To Issue 138
Citation: Metzmann F, Muenzer C, “Advanced Delivery Device Technology to Simplify the Reconstitution of Lyophilised Drugs”. ONdrugDelivery, Issue 138 (Oct 2022), pp 77–82.
Fred Metzmann and Chris Muenzer present Haselmeier’s innovative approach to the development of reconstitution devices that allow easy and convenient administration of freeze-dried biologics.
“Reconstitution devices based on Haselmeier’s reusable and disposable pen platform technology empower patients and caregivers to inject their medication accurately with minimised risk of use errors.”
Lyophilisation (freeze drying) is a proven process for increasing the shelf life of vaccines, biologics and other injectables. It allows for room temperature storage of the drug product, which can be beneficial for transportation and distribution, especially in countries that lack sufficient cold chain infrastructure. However, the reconstitution of these products requires several use steps that make them challenging to administer, particularly by patients and caregivers in non-clinical settings.
Reconstitution devices based on Haselmeier’s reusable and disposable pen platform technology empower patients and caregivers to inject their medication accurately with minimised risk of use errors. Additionally, these solutions support rapid and sustainable customisation, with the potential to significantly accelerate the development of combination products for customers.
BIOLOGICS ARE ON THE RISE – INJECTABLES LEAD VALUE SIZE AND GROWTH
The emerging prevalence of chronic diseases leads to a steady rise in the annual number of approvals for various types of biologics including monoclonal antibodies, cell therapies, recombinant proteins, peptides, vaccines and gene therapies. Innovative therapies and innovations in biologics are improving patient outcomes in conditions such as hepatitis C, cancer, autoimmune diseases, metabolic disorders, cardiovascular diseases and orphan diseases.1,2
The majority of these biologics are currently administered through intravenous infusion and typically administered in a clinical setting (hospitals/infusion centres). However, subcutaneous injections of biologics are increasingly preferred over intravenous infusions because they reduce the burden on healthcare providers and payers by taking much less time and offering a lower risk of complications (infections, infusion reactions and others).3,4
In addition, recent advances in the delivery of traditional biologics include methods to increase the acceptable volume of drug solutions that can be administered subcutaneously.
Thus, more than half the drugs approved by the US FDA and EMA in the first half of 2021 were injectables, with subcutaneous and intramuscular products accounting for an increasing share.5,6
In addition, more than 3,000 biologic therapeutics are currently in clinical trials worldwide, representing a ratio of approximately 50% biologics to small molecules in all Phases I–III.7
“While liquid formulations are typically preferred, lyophilisation is an important alternative for making dry biopharmaceutical formulations achieve a longer shelf life.”
LYOPHILISATION TO ENHANCE SHELF LIFE OF BIOLOGICS
Biological drugs are inherently less stable in liquid formulations and tend to lose efficacy due to alterations in their pharmacokinetic and pharmacodynamic properties. This can make it challenging to preserve them for longer periods.
While liquid formulations are typically preferred, lyophilisation is an important alternative for making dry biopharmaceutical formulations achieve a longer shelf life.
Lyophilisation is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapour without passing through a liquid phase. The lyophilisation process can be used to stabilise formulations and therapeutic molecules in a commercially validated manner. In contrast to liquid formulations, the solid obtained by this process has a greater degree of stability and can be stored for longer periods of time at higher temperatures, such as outside of refrigeration.8
NEED FOR RECONSTITUTION SYSTEMS PRIOR TO INJECTION
Before it can be injected, a lyophilised drug must be reformulated into a liquid form by mixing with a suitable diluent. This process of reformulation is known as drug reconstitution, which requires additional preparation steps.
Typically, the drug product is supplied in glass vials with rubber plugs and is mixed or reconstituted with a diluent (usually 5% dextrose solution, normal saline, bacteriostatic water or sterile water for injection) before administration. An incompletely dissolved product can be hazardous to the patient; therefore, reconstitution is a critical performance parameter for lyophilised products.
CONVENTIONAL RECONSTITUTION PROCESS
Traditionally, the reconstitution process is a multi-step procedure, which can take up to 30 minutes in some cases. The process requires interaction with a reconstitution kit, usually consisting of several pieces of equipment, including but not limited to a medication vial, a diluent vial, a syringe, a reconstitution needle and an injection needle (Figure 1).
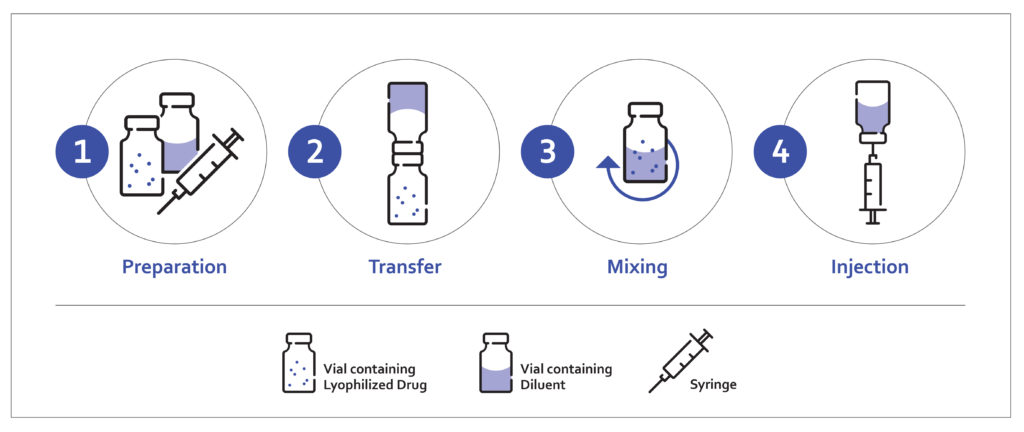
Figure 1: Conventional reconstitution process.
The process typically starts with the manual extraction and transfer of the diluent from the first vial to the second vial containing the lyophilised product by using a syringe and transfer needle. After transferring, the contents are mixed until the final mixture is fully reconstituted. The process is usually performed by a trained healthcare provider or caregivers and requires the use of vial adaptors and vial-to-vial systems.
After the drug product has been reconstituted, it must be withdrawn into a syringe and injected. This process can introduce air bubbles into the solution and is dependent on the skill of the user to prepare an accurate dose. One study using 1 mL syringes found that there was “a significant variance in the accuracy, precision and repeatability to prepare a proposed dose”.9
“With one approved product and several more in development, Haselmeier has experience with multiple solutions that allow easy and convenient administration of freeze-dried biologics.”
GROWTH DRIVERS AND CHALLENGES
The lack of easy-to-use drug reconstitution delivery systems has limited market opportunities in the past. However, with the introduction of novel drug reconstitution systems, the delivery of lyophilised drugs has become convenient. Such systems provide better treatment options for patients and save time and labour for caregivers. With the availability of these systems, lyophilised formulations can be considered a viable alternative to liquid formulations.
PATIENT-CENTRIC DEVELOPMENT
Regulatory requirements are among the biggest challenges facing pharmaceutical companies and device development and manufacturing organisations, especially for products offered as combination products. Global regulatory authorities continue to place great emphasis on ensuring quality and risk mitigation. But they are also paying increasing attention to the development of drug delivery devices that focus on ease of use and patient safety to promote sustained treatment adherence.
For a successful path to approval, essential performance requirements for a therapy’s delivery technology must be defined early in the development process. This helps to ensure that the development generates all necessary data required for submitting the application to a regulatory authority.
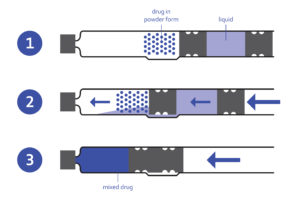
Figure 2: Dual-chamber cartridge.
BOX 1: DUAL-CHAMBER CARTRIDGE SYSTEMS
These container closure systems are designed to enhance the portability, efficiency and optimum delivery of the lyophilised drugs. The components (powder and liquid) are stored safely, can be reconstituted very easily and are then administered safely and conveniently.
Dual-chamber cartridges require the use of injection pens and can combine either liquids with liquids or liquids with medications in powder form. The two chambers are separated by a plunger. Instead of deploying two containers to store the lyophilisate in one and the diluent in another, the dual-chamber cartridges allow mixing and application to be carried out in one operation. The pen system enables higher dosing accuracy and a lower risk of contamination. This translates into higher safety for patients, as literally just the push of a button is needed (Figure 2).
HASELMEIER’S SOLUTIONS FOR AN EASIER AND MORE CONVENIENT RECONSTITUTION
An injection system for self-administration combines engineering and design to ensure the safe administration of therapy. This needs to be balanced with a system that removes both the potential physical and emotional barriers to patient adherence to therapy.
With one approved product and several more in development, Haselmeier has experience with multiple solutions that allow easy and convenient administration of freeze-dried biologics (Figure 3). The company’s approach is based on proven platform technologies for both disposable and reusable injection pen devices.
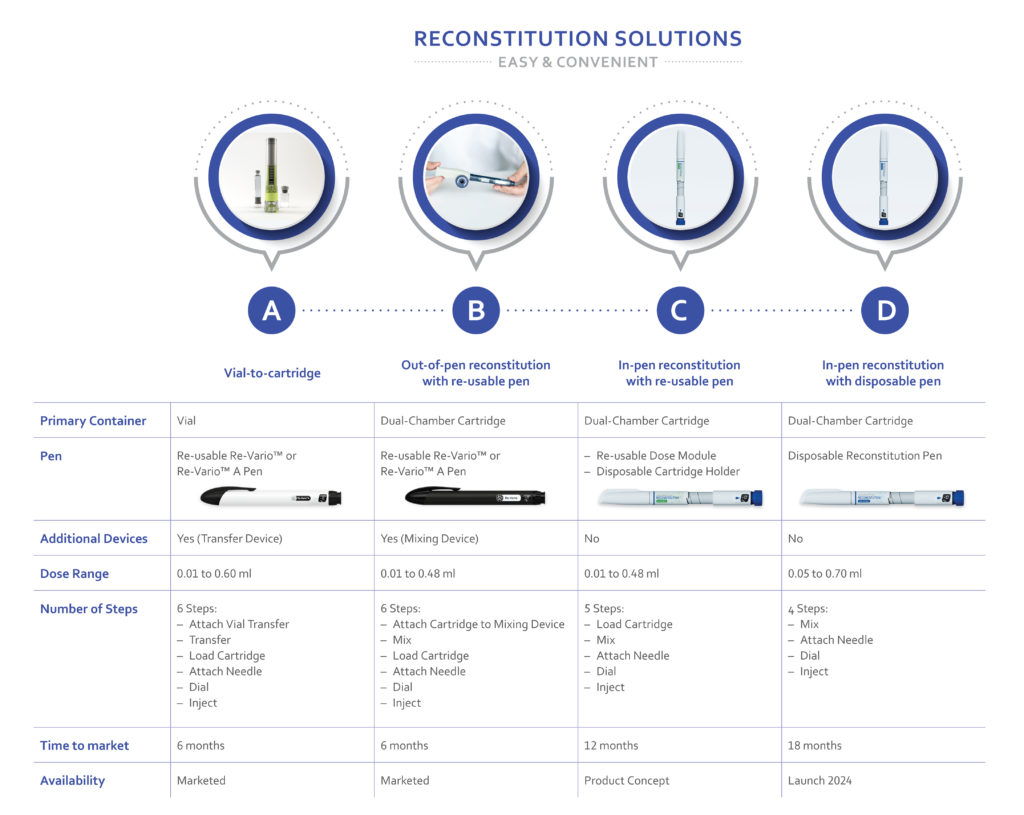
Figure 3: Overview of reconstitution systems by Haselmeier. Showed transfer device (solution A) and mixing device (solution B) and all associated intellectual property, including pictures shown, are owned by Duoject Medical Systems Inc.
These solutions can be separated into two categories based on the primary drug container. For vial-based products, a vial-to-cartridge solution is available. The alternative is to fill drugs into dual-chamber cartridge systems, which allows several other options.
All these solutions use a precision mechanism to deliver the drug product from a cartridge. The injection pen allows the user to rotate a knob until their exact dose is displayed, which provides a simpler user experience than withdrawing drug from a vial. In addition, the use of this type of mechanism can reduce the variability in the delivered dose.10
Solution A: Vial-to-Cartridge System
For vial-based products, the use of a reusable injection pen, along with a vial-to-cartridge reconstitution system, can simplify the delivery without the need to change the fill-finish process. In this solution, a system like Duoject Medical Systems’ PENPREP EVO™* allows the reconstitution of solid-form drugs in vials with standard diluent-filled cartridges. The final result is a filled cartridge that the user can place into a reusable pen, such as the Haselmeier Re-Vario™ A. The combination of these two marketed technologies reduces the number of use steps, simplifies the transfer process and may have a positive impact on the accuracy of the injection.** In addition, this approach uses established vial lyophilisation infrastructure, which reduces the risk of capacity constraints that can jeopardise time to market.
DUAL-CHAMBER CARTRIDGE SYSTEMS
A drug product can be filled into a dual-chamber cartridge system (explained in Box 1). Once the drug is available in this format, Haselmeier has three different solutions:
Solution B: Out-of-Pen Reconstitution with Reusable Pen
For this solution, the patient is supplied with a separate mixing device used to reconstitute the drug product. Once the mixing step is completed, the cartridge is loaded into the reusable pen and the drug can be easily injected. The advantage of out-of-pen reconstitution is that it simplifies the injection pen itself, but it is at the disadvantage of having another reusable device that the patient needs to manage. The use of in-pen reconstitution technology can reduce this burden.
Solution C: In-Pen Reconstitution with a Reusable Pen
In this solution, each drug cartridge is supplied with a disposable cartridge holder with a built-in reconstitution mechanism. The patient attaches this cartridge holder to the pen and rotates the mechanism to mix the drug product. Once the mixing step is completed, the drug can be injected with no additional steps. After the cartridge is empty, it is removed along with the cartridge holder and disposed. This solution eliminates the need for a separate mixing device but adds additional cost for the disposable cartridge holder.
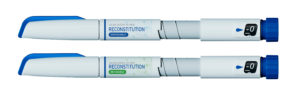
Figure 4: Examples of Haselmeier’s disposable and reusable versions of the In-pen reconstitution solutions.
Solution D: Disposable Pen with Integration Reconstitution
The simplest solution for the patient is to provide a disposable device with an already-integrated drug cartridge. With this device, the patient simply completes the mixing step and then injects. After use, the complete pen is disposed. This solution minimises the user steps and the need to manage a reusable device at the expense of reduced sustainability (Figure 4).
INTELLECTUAL PROPERTY POSITIONING
Part of Haselmeier’s intellectual property (IP) strategy is to constantly maximise and expand patent protection for its injection pen technology through continuous innovation and new applications. Haselmeier has already filed several patent applications for its injection pen solutions, covering various innovative technologies and safety features.
CUSTOMISATION OPTIONS
In addition to the options above, Haselmeier can provide custom solutions to meet the unique needs of any patient population or drug product. The company has experience in developing unique devices that still benefit from its broad IP portfolio. This can range from matching brand colours to custom container closures, to additional features like reuse prevention or needle safety. In addition, its flexible manufacturing operations for both low-volume manual assembly and high-volume fully automated assembly can fulfil different market requirements.
ADVANTAGES OF ADVANCED IN-PEN RECONSTITUTION SYSTEMS FROM HASELMEIER
Haselmeier continues to develop innovative drug delivery device technology platforms with the patient in mind. These reconstitution systems offer multiple benefits to patients as well as biopharmaceutical and pharmaceutical companies, enabling them to bring lyophilised therapeutics to the market successfully (Figure 5).
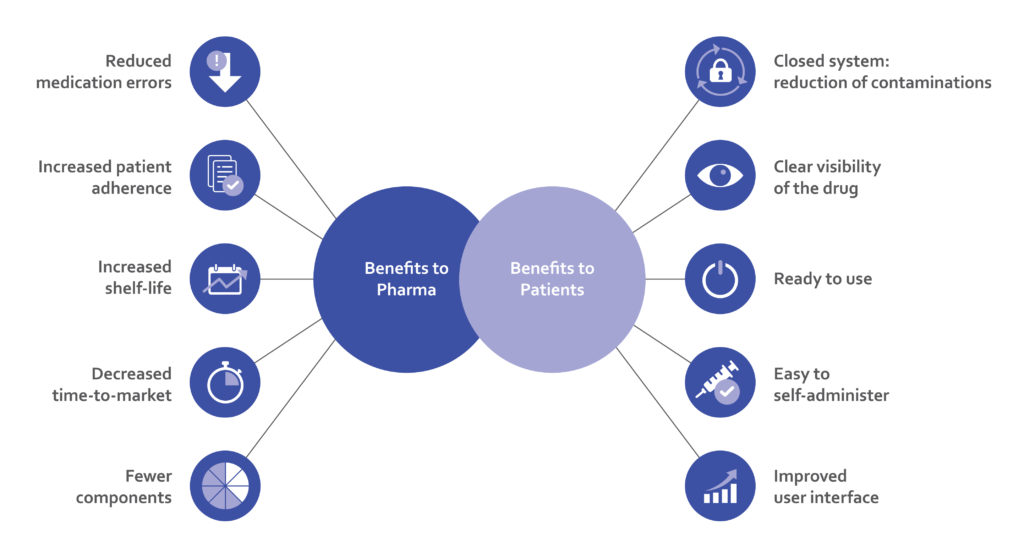
Figure 5: Advantages of advanced in-pen reconstitution systems for pharma companies and patients.
*Penprep Evo is a trademark of Duoject Medical Systems and is not affiliated with Haselmeier.
**Duoject is neither affiliated with Haselmeier GmbH nor endorses the use of its products.
Disclaimer
The products shown in this article are under development and some of them may not yet have been approved for sale under applicable medical devices regulation. The content provides general information about these products, their fields of application and intended use. Their function, performance and specifications are subject to customer-specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice.
REFERENCES
- “Global Biologics Market: Industry Analysis and Forecast (2021-2027) by Product, Application, and Region Research Report, Maximize Market Research, Oct 2021.
- Walker N, “Biologics: Driving Force in Pharma”. Pharma’s Almanac, Jun 5, 2017.
- Aitken M, “Biologics Market Dynamics: Setting the Stage for Biosimilars”. FDA/FTC Workshop on a Competitive Marketplace for Biosimilars, Mar 9, 2020.
- Betti CJ, Silva RM, Doukas ME, “Blockbuster Biologics Review Quarterly Update – April 2020”. PDF, Morgan Lewis, accessed Sep 2022.
- “Biopharma: Biopharmaceutical Products in the U.S. and European Markets, U.S. Approvals, 2002-Early 2021”. Biopharma.com, accessed Sep 2022.
- “EFPIA Pipeline Review 2021 Update, Project Report”. PDF, efpia.eu, Feb 2021.
- “New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products”. US FDA, accessed Sep 2022.
- “Rising Demand for Lyophilized Products”. contractpharma.com, accessed Sep 2022.
- Meyer CH et al, “Accuracy, precision and repeatability in preparing the intravitreal dose with a 1.0-cc syringe”. onlinelibrary.wiley.com, Apr 7, 2011.
- Asakura T et al, “A comparison of the handling and accuracy of syringe and vial versus prefilled insulin pen (FlexPen)”. Diabetes Technol Ther, 2009 Vol 11(10), pp 657–661.

