Citation: Hasegawa H, Suziki T, “Advances in Multilayer Technology for Wearable Injector Primary Packaging. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 30-33.
Hiroki Hasegawa and Tomohiro Suzuki discuss how multilayer technology used in the drinks bottle industry can be used to create syringes and vials for biologics, avoiding problems such as oxidation and absorption that occur with other materials.
Improving the quality of plastic vials and syringes is an important aspect of ensuring that wearable devices deliver biologic drugs in the best possible condition to patients. This includes preventing oxidation of the drugs and absorption of unwanted particles.

Figure 1: OXYCAPT™ Vial & Syringe.
Mitsubishi Gas Chemical (MGC), one of the Mitsubishi companies, is a leading company in the field of oxygen barrier and absorbing technologies. It has created an innovative polymer, Nylon-MXD6, which has been used for the middle layer of multilayer beverage bottles for many years to prevent oxidation and evaporation of carbon dioxide from drinks. The company also developed an oxygen absorber, AGELESS®, that has been used for IV solutions and prefilled syringes to prevent oxidation of injectable drugs for more than 30 years.
Based on these technologies and experiences, we have successfully developed multilayer plastic vials and a syringe called OXYCAPT™ (Figure 1).
OXYCAPT™ consists of three layers; the drug contact layer and the outer layer are made of cyclo olefin polymer (COP), and the oxygen barrier layer is made of our novel polyester (Figure 2).
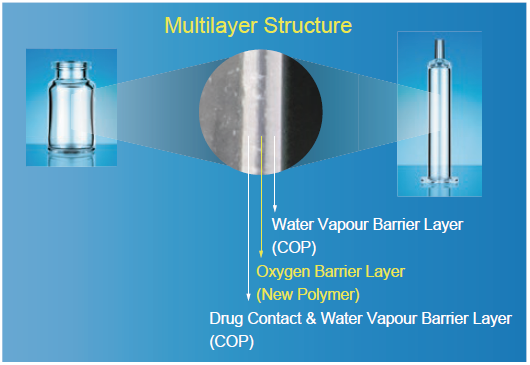
Figure 2: The multilayer structure of OXYCAPT™.
BENEFICIAL FEATURES OF OXYCAPT™
The OXYCAPT™ Multilayer Plastic Vial & Syringe can offer a range of benefits including:
- An excellent oxygen barrier
- A high water vapour barrier
- An excellent ultraviolet ray barrier
- Very low extractables
- High pH stability
- Low protein adsorption and aggregation
- A silicone-oil free barrel
- High transparency
- High break resistance
- Easier disposability
- Lighter weight.
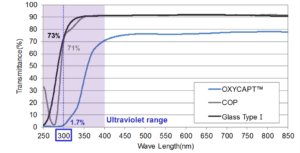
Figure 3: Percentage of light transmitted through the UV barrier.
OXYCAPT™ also has excellent UV barrier properties. Specifically, whereas about 70% UV light of 300 nm transmits through glass and COP, only 1.7% transmits through OXYCAPT™ (Figure 3). This feature also contributes to the stability of biologics.
PRODUCT DETAILS
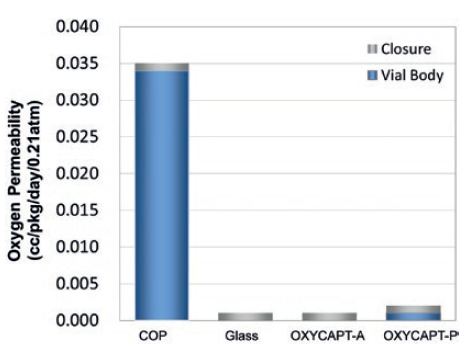
Figure 4: Oxygen permeability of the types of OXYCAPT™.
There are two types of OXYCAPT™, OXYCAPT™-A and OXYCAPT™-P. OXYCAPT™-A is suitable for drugs that are especially oxygen sensitive as it has a glass-like oxygen barrier (Figure 4) and an oxygen-absorbing function. According to some internal studies, this function gives OXYCAPT™-A a lower oxygen concentration in headspace than Type 1 glass. OXYCAPT™-P is recommended for all drugs as, although there is no oxygen-absorbing function, the oxygen barrier of OXYCAPT™-P Vial is about 20 times better than that of COP monolayer vial.
In terms of the water vapour barrier, OXYCAPT™ is not equal to the performance of glass. However, it is similar to COP, which has been used for injectable drugs for a long time, and easily meets the requirements of a water vapour barrier for the ICH guideline.
OXYCAPT™ Syringe consists of a tip cap, barrel, PTFE-laminated stopper and a plunger rod. Although a very small amount of silicone oil is sprayed on the stoppers of the syringe, no silicone oil is baked on the barrel. According to our internal studies using existing antibody, we have found this feature leads to much less protein aggregations compared to existing Type 1 glass syringes.
OXYCAPT™ Vial & Syringe are produced by co-injection moulding technology. Although this technology has been applied to beverage bottles for many years, we are the first company that has succeeded in using it to develop multilayer plastic syringes. We have also developed inspection methods for the oxygen barrier layer. All of the containers are 100% inspected by state-of-the-art inspection machinery.
MGC can offer bulk vials, ready to use (RTU) vials and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-based nest and tub formats (Figures 5 & 6). The nests and tubs are usually sterilised by gamma ray. There are 2 mL, 6 mL, 10 mL and 20 mL options for the vials, and 1 mL “long” and 2.25 mL for syringes. We are happy to provide some samples for initial testing, free of charge.
Figure 5: The nest and tub format for vials.
Figure 6: The nest and tub format for syringes.
Each polymer meets the requirements of USP661, USP87, USP88, EP, and has been filed in the US FDA’s Drug Master File (DMF). The syringes are produced and controlled in accordance with ISO 13485.
RESEARCH EVIDENCE
Studies have shown extremely low extractables with OXYCAPT™. One study was conducted to confirm volatile, semi-volatile and non-volatile impurities from OXYCAPT™. Water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were used and impurities were measured by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with control, no impurities were detected in any of the OXYCAPT™ containers.
The second study was conducted to confirm inorganic extractables from OXYCAPT™. The levels of extractables were similar to those from COP, which is well known as an extremely pure polymer, and less than Type 1 glass. The smaller amount of inorganic extractables contributes to the stability of the pH in drugs (Figure 7).
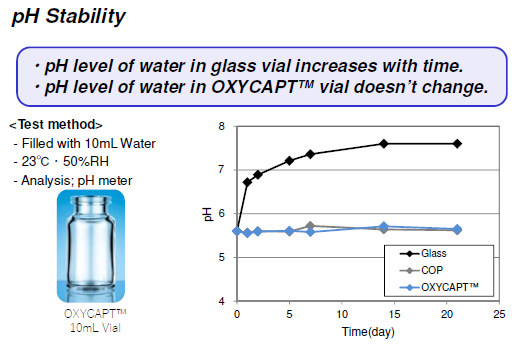
Figure 7: The pH stability of OXYCAPT™ versus water.
Recently, we also investigated the ability of OXYCAPT™ to withstand cold storage. OXYCAPT™ vials and a competitor’s COP monolayer vials were stored at approximately -180˚C, using liquid nitrogen, and all the vials were dropped from a height of 150 cm. Although some COP monolayer vials were then broken, no breakage was observed in OXYCAPT™ vials (Figure 8). As liquid nitrogen storage has gradually become more popular thanks to the spread of regenerative medicine, it is expected that OXYCAPT™ could benefit from this development.

Figure 8: Suitability of OXYCAPT™ to cold storage.
CONCLUSION
OXYCAPT™ has been developed to solve some of the problems facing the pharmaceutical industry in creating syringes and vials suitable for wearable devices. In addition to special features of COP such as a high water vapour barrier, high break resistance, very low extractables and low protein absorption, OXYCAPT™ can offer a high oxygen and UV barrier. We believe OXYCAPT™ definitely brings a lot of benefits which could contribute to improving patients’ lives.

