To Issue 172
Citation: Vandewalle J, Holden C, “Advancing Subcutaneous Infusion Therapy for Patients Through Innovation and Partnerships”. ONdrugDelivery, Issue 172 (May 2025), pp 8–11.
Julien Vandewalle and Clare Holden explain Convatec’s long-term partnerships with pharmaceutical and medical device companies are driving development in chronic care by introducing subcutaneous technologies that support the treatment of conditions such as diabetes and Parkinson’s disease – showcasing Convatec’s Neria™ Guard infusion set.
In 2024, a video of a 53-year-old man from the UK with advanced Parkinson’s disease went viral. It was a before-and-after video that captured what he called the “extraordinary and life-changing” improvement from a new infusion therapy.1 The two-minute video showed him preparing a cup of tea one-handed and with severe shaking. The film then cuts to the man smoothly scooping out his tea bag and walking to the kitchen dustbin to throw it away with ease – his progress gripped viewers and made headlines around the world, and rightly so.
Relief for this patient came through PRODUODOPA® (foslevodopa/foscarbidopa), a drug from Convatec’s partner AbbVie, delivered via a portable infusion pump and Convatec’s Neria™ Guard infusion set. The infusion set specifically provides continuous drug delivery, which helps to achieve stable symptom control, enabling patients to regain their independence.
This story is just one of the powerful examples of how Convatec is driving innovation in infusion sets and working with leading pharmaceutical partners to make a tangible and positive impact on people’s lives worldwide.
CUSTOMER CENTRICITY AT THE CORE
More than one million people around the world rely on Convatec infusion sets every day (Figure 1). Convatec has long recognised the opportunity to provide a better solution for patients with Parkinson’s disease and other chronic conditions by developing an infusion set that works seamlessly with a pump to deliver medication in a controlled manner, featuring an easy-to-use design that supports high-quality care that can be delivered anywhere and everywhere.
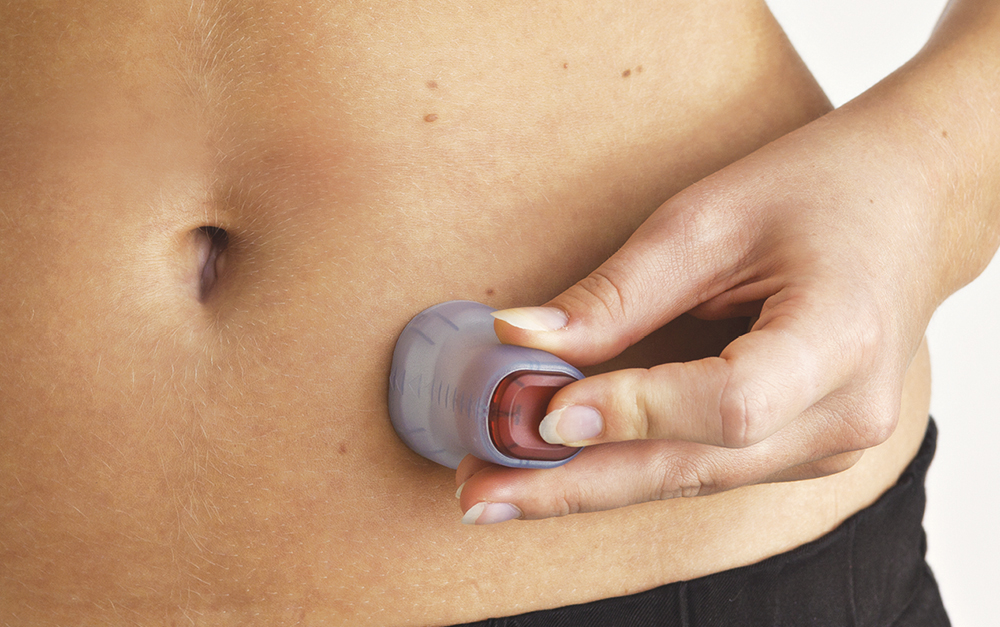
Figure 1: Neria™ Guard infusion set in use.
Convatec collaborates closely with its partners to tailor solutions that align with the specific needs of their drugs and therapy areas. By prioritising user-centric development, Convatec ensures that its infusion sets meet the highest usability standards, optimising the patient experience and safety.
“CONVATEC SUPPORTS PHARMACEUTICAL COMPANIES IN BRINGING NOVEL SUBCUTANEOUS THERAPIES TO MARKET, EXPANDING TREATMENT OPTIONS FOR PEOPLE WHO MAY NOT TOLERATE ORAL MEDICATIONS EFFECTIVELY.”
For Parkinson’s disease and other chronic conditions, Convatec supports pharmaceutical companies in bringing novel subcutaneous therapies to market, expanding treatment options for people who may not tolerate oral medications effectively. These partnerships also help the company to expand its global reach.
Convatec’s collaboration with AbbVie is one of the many examples of how pharmaceutical and medical device companies can work together to optimise subcutaneous drug delivery.
AbbVie’s foslevodopa/foscarbidopa formulation is marketed in selected EU countries as PRODUODOPA® and is available for use with Convatec’s Neria™ Guard infusion set. The drug has recently been approved by the US FDA as VYALEV™, where the Neria™ Guard infusion set is approved for subcutaneous infusion of medication.
This therapy is the first and only subcutaneous 24-hour infusion of a levodopa-based therapy for the treatment of advanced Parkinson’s disease in the US.2,3
Convatec’s Neria™ Guard infusion set, used together with a portable infusion pump and foslevodopa/foscarbidopa, plays a critical role in this therapy, ensuring a consistent drug delivery process.
A LEADER IN SUBCUTANEOUS INFUSION SETS
Guided by Convatec’s vision – pioneering trusted medical solutions to improve the lives we touch – Convatec’s infusion set technologies support patients who rely on subcutaneous therapies for conditions such as diabetes mellitus, Parkinson’s disease, pain management therapy, primary immune deficiency and thalassemia.
Kjersti Grimsrud, President & Chief Operating Officer, Infusion Care, at Convatec said, “At Convatec, we are focused on supporting people living with chronic conditions. Our pioneering subcutaneous infusion set technology, Neria™ Guard, is enabling a wide range of pharmaceuticals and medical device companies to extend access and improve the lives of people counting on us.”
SUBCUTANEOUS DRUG DELIVERY DEVICES
The shift from intravenous infusion to subcutaneous administration is gaining momentum. In this evolving landscape, infusion sets can play a crucial role in optimising subcutaneous drug delivery by enhancing ease of administration, improving the patient experience and supporting the broader adoption of subcutaneous therapies.
For subcutaneous drug delivery, infusion sets can be flexible in drug volume and do not have volume requirement limitations, unlike other medical devices. Furthermore, infusion sets, in combination with a pump system, can be an ideal option for medications that require continuous delivery in a controlled manner. With so many potential scenarios and drug requirements, infusion sets with user-friendly features, such as easy insertion, are one of the ideal choices for subcutaneous drug delivery devices.
ALL-IN-ONE INFUSION SET
Neria™ Guard is an all-in-one infusion set with automatic inserter, designed to support simplicity for both at-home and outpatient treatment, with features such as automatic insertion with the touch of a button. The infusion set connects to a pump at one end and the user’s body at the other end to deliver medication into the subcutaneous tissue continuously.

Figure 2: Neria™ Guard infusion set.
Studies indicate that Neria™ Guard’s easy and intuitive insertion technique protects against user errors (internal company data), may help support at-home use and encourages independence in patients’ everyday lives (Figure 2).4
“CONVATEC WORKS WITH LEADING PHARMACEUTICAL AND MEDICAL DEVICE COMPANIES TO INTEGRATE ITS INFUSION PRODUCTS SEAMLESSLY INTO COMPREHENSIVE DRUG DELIVERY SOLUTIONS.”
Convatec works with leading pharmaceutical and medical device companies to integrate its infusion products seamlessly into comprehensive drug delivery solutions. The company’s capabilities and know-how, developed over more than 30 years, are highly transferable to other technologies and therapies, such as insertion technologies, fluid pathways, infusion cannulas, design for manufacturing and process knowledge for high-scale manufacturing.
Convatec’s lasting relationships with existing partners and its commitment to quality are proof that the company is devoted to building strong partnerships, believing that transparency and proactivity are essential for productive relationships. Convatec’s partners and collaborators include AbbVie, Beta Bionics (Irvine, CA, US), Medtronic (Minneapolis, MN, US), Mitsubishi Tanabe (Osaka, Japan), SOOIL (Seoul, South Korea), Tandem Diabetes Care (San Diego, CA, US) and Ypsomed.
Convatec believes that collaborating with more medical device and pharmaceutical companies will help people with chronic conditions to live more fulfilling lives.
REGULATORY REQUIREMENTS AND MARKET READINESS
Bringing a medical device to market requires strict adherence to regulatory requirements and standards to ensure patient safety, efficacy and drug-device compatibility. This is an increased focus area in the industry as shown by the EU’s Medical Device Regulation (MDR) and the FDA’s Center for Devices and Radiological Health.
The production of Neria™ Guard adheres to the ISO 13485:2016 quality standard, meeting industry benchmarks for subcutaneous drug delivery systems, guaranteeing reliability and precision. The infusion set already meets the new MDR requirements in the EU and is also approved in the US with a 510(k) as a Class II medical device. Convatec also has market approval for the Neria™ Guard in many other countries worldwide.
LARGE-SCALE MANUFACTURING AND CONTINUOUS INVESTMENT
Across its three infusion set manufacturing sites, Convatec produces approximately 130 million infusion set devices each year. Convatec’s manufacturing capabilities include manual, semi- and fully automated manufacturing, as well as extrusion and injection moulding (Figure 3).
Figure 3: Video showing manufacturing of Convatec’s Neria™ Guard.
Convatec continues to invest in its manufacturing capabilities and footprint. In 2022, Convatec Infusion Care doubled the manufacturing capacity at its manufacturing site in Denmark, making it the most advanced manufacturing site in the overall Convatec network.5 The company has continued to invest in its Infusion Care manufacturing, adding capacity to be able to meet demand, and it has more than doubled R&D investment in recent years6 with the strongest innovation pipeline in its history.
DOING WHAT IS RIGHT FOR ALL STAKEHOLDERS
Convatec continues to invest in R&D to support innovation in infusion therapy, while integrating responsible business practices. The company’s sustainability efforts seek to drive progress towards Convatec’s vision, while generating value for stakeholders. For example, innovative infusion sets such as the Medtronic Extended infusion set, which is the first and only infusion set approved for up to seven days of wear (versus three days for standard sets), reduces the environmental footprint of plastic waste from insulin pump therapy. Developed in partnership with Convatec, this infusion set is estimated to reduce plastic waste by up to 50%.7 Innovation like this is important for Convatec to reach its goal to achieve net zero by 2045.
Sustainability in the company’s operations is also important. As of 2024, renewable electricity accounts for 100% of total electricity consumed at its manufacturing sites.8 By minimising waste, expanding renewable energy and optimising manufacturing processes, Convatec seeks to continue delivering innovative solutions responsibly.
A FOREVER CARING PARTNER
Convatec’s promise – forever caring – is core to its business. The company has revolutionised the field of subcutaneous infusion therapy for patients by tackling essential challenges encountered by both patients and healthcare professionals. Its solutions have made a notable difference in managing conditions such as diabetes mellitus, Parkinson’s disease, pain, primary immune deficiency and thalassemia.
Convatec is more than an infusion sets manufacturer; it is a trusted partner on the journey towards better health and improved lives, and welcomes collaboration and strategic partnerships to bring impactful innovation to life.
MRL: AP-74271-GBL-ENG-v3
REFERENCES
- “Video shows drug’s ‘life-changing’ effect on man with Parkinson’s”. BBC News, Jul 2024.
- “US FDA Approves VYALEV™ (foscarbidopa and foslevodopa) for Adults Living with Advanced Parkinson’s Disease”. Press Release, Abbvie, Oct 2024.
- “Convatec collaborates with AbbVie for VYALEV™ therapy for the treatment of advanced Parkinson’s disease in the United States”. Press Release, Convatec, Oct 2024.
- Hillman E, “Examining the use of sharp-free subcutaneous infusion devices with apomorphine”. BJNN, 2020, Vol 16(4), pp 2–6.
- Convatec Annual Report, 2022, accessed Apr 2025.
- Convatec Annual Report, 2024, accessed Apr 2025.
- “Medtronic Launches World’s First and Only Infusion Set for Insulin Pumps that Doubles Wear Time up to 7 days in U.S.” Press Release, Medtronic, Nov 15, 2022.
- Convatec Annual Report, 2023, accessed Apr 2025.

