 Citation: Panigone S, Sandri F, Nicolini G, “An Environmentally Sustainable, Patient-Centred Solution for Asthma & COPD”. ONdrugDelivery Magazine, Issue 106 (Apr 2020), pp 14-19.
Citation: Panigone S, Sandri F, Nicolini G, “An Environmentally Sustainable, Patient-Centred Solution for Asthma & COPD”. ONdrugDelivery Magazine, Issue 106 (Apr 2020), pp 14-19.
Sara Panigone, Federica Sandri and Gabriele Nicolini provide an analysis of carbon-minimal pMDIs for asthma and COPD, stressing the importance of balancing environmental sustainability with therapeutic efficacy to provide a sustainable and patient-centred solution.
“The most commonly used inhaler in Europe is the pMDI7, which relies on the driving force of propellants.”
Globally, chronic pulmonary conditions cause a significant burden, and are among the leading causes of morbidity and mortality.1 Asthma and chronic obstructive pulmonary disease (COPD) are the most common chronic pulmonary diseases; it is estimated that there are at least 300 million patients with asthma and 250 million patients with COPD worldwide.2-4 Approximately 3.2 million and 400,000 deaths are attributable to COPD and asthma each year, respectively.1 COPD is currently the third leading cause of death worldwide, with the burden expected to increase further within the next 10 years.3,5
Despite this, chronic respiratory diseases are often overlooked compared with other major causes of morbidity and mortality.6 No cure exists for either COPD or asthma; both conditions are primarily managed with chronic use of inhaled therapies delivered via an inhaler. Three main types of handheld inhaler are available – pressurised metered dose inhalers (pMDIs), dry powder inhalers (DPIs) and soft mist inhalers (SMIs).7 Choosing the most suitable inhaler for each patient is as important as choosing the most appropriate drug, as patient preference and ability to use a device may influence adherence to treatment.7 The most commonly used inhaler in Europe is the pMDI (Figure 1)8, which relies on the driving force of propellants to atomise droplets containing drugs for deposition in the lungs.9

Figure 1: Proportion of inhalers sold by device type in 16 European countries from 2002–2008.8 (“Liquids” refers to nebulised formulations.)
ENVIRONMENTAL IMPACT OF INHALERS
Annually, an estimated 800 million pMDIs are manufactured globally, using more than 11,500 tonnes of propellants.10 Chlorofluorocarbons (CFCs) were also used as propellants for pMDIs until 1989, when the Montreal Protocol banned the use of CFCs as ozone-depleting substances in order to prevent further damage to the ozone layer.11 This prompted a global, industry-wide transition from CFC propellants; in the case of pharmaceutical products, this translated into a progressive switch towards non-ozone-depleting hydrofluorocarbon (HFC) propellants (also known as hydrofluoroalkane (HFA) propellants) specifically HFA 134a and HFA 227ea.
Since CFC production for manufacturing pMDIs peaked in 1997 at approximately 10,000 tonnes, the transition from CFCs to HFCs led to a 97% reduction to approximately 300 tonnes in 2013, significantly reducing the carbon emissions associated with propellant use in pMDIs.12 A number of companies, including Chiesi, executed the move from CFC to HFC pMDIs, including to HFA 134a, which has the lowest global warming potential (GWP) of all propellants approved for pharmaceutical use.13
PATIENT BENEFITS ASSOCIATED WITH HFCs
In addition to the reduced environmental impact of HFC pMDIs compared with CFC pMDIs, other technical advancements with HFC pMDIs led to improved patient outcomes. CFC pMDIs were suspension based and required shaking before use; this heterogeneity often caused dose variability.14 Moreover, CFC suspension formulations needed to be delivered with a relatively large device aperture diameter to avoid blockage. This led to higher velocity and lower duration of the aerosol plume, resulting in increased drug deposition in the oropharynx.14 Additionally, the relatively large particles – 3.5 μm mass median aerodynamic diameter (MMAD) – aerosolised by suspension-based CFC pMDIs did not reach the small airways (≤2 mm in diameter).15 However, it is well known that dysfunction of the small airways is linked to symptoms in patients with COPD or asthma.16,17
“pMDI inhalers account for a very small proportion (≤0.1%) of global emissions.20,23”
Technical advancements have enabled some drugs to be dissolved within the HFC propellant. Alongside the advent of HFC suspension-based pMDIs, this also led to the introduction of solution-based pMDIs, a homogeneous solution that does not require shaking before use. Such solutions are compatible with devices with smaller aperture diameters, leading to lower velocity and higher duration of the aerosol plume.14
Specific formulation technologies, such as Chiesi’s Modulite technology, have enabled the related solution-based pMDIs to be tailored for extra-fine drug delivery, reducing the particle size of emitted aerosol (<2 μm MMAD) and thereby allowing deeper penetration in the bronchial tree, effectively reaching both large and small airways.14 The reduction in particle size, lower velocity and higher duration of aerosol plume in such HFC solution-based pMDIs also facilitates patient co-ordination between actuation of the device and inhalation, which is a common obstacle with the use of pMDIs.14
Pharmacokinetic data also showed that with a dose from a solution-based HFC pMDI that uses Modulite technology (which is 2.5 times lower than from a CFC-based pMDI), pulmonary absorption was 86% higher and systemic exposure was 35% lower than a CFC-based pMDI, resulting in less cortisol suppression.18 In addition, HFC pMDIs do not result in dose loss when stored inverted or in a cold climate and have significantly lower dose variability at the end of each canister’s life compared with CFC pMDIs.15
CONTROVERSIES SURROUNDING HFCs
“Many expert respiratory healthcare providers have expressed concern that implementation of a device switch initiative may lead to detrimental effects on quality of care and patient outcomes.”
As concerns over climate change have grown in recent years, the general industrial use of HFC propellants is now the object of a phasing-down strategy agreed by EU Regulation No 517/2014 and the Kigali Amendment to the Montreal Protocol.19,20 The aim of this phasing down, which has already started in Europe, is to encourage use of low GWP alternatives and to reduce consumption and emissions of high GWP HFCs. Currently, the EU regulation recognises an exemption for HFCs for pharmaceutical use, including pMDIs.19 However, in some countries, governments have started actions to assess the contribution of pMDIs to total CO2 emissions and to propose short-term solutions.
There is growing interest from some healthcare systems that a reduction in HFC emissions could be primarily achieved by reducing use of pMDIs and increasing use of DPIs, which are propellant free. As an extreme example, the UK has taken a radical approach, stating that the NHS should aim to reduce the impact of respiratory treatments by 50% before 2022 by increasing prescriptions of low GWP inhalers.21 This approach was supported by data that showed switching to DPIs from pMDIs would result in large carbon savings.22 This has proven controversial, since fluorinated-gas (F-gas) usage only accounts for 2.2% of total annual greenhouse gases emissions – and refrigerators and air conditioning units contribute to the majority of F-gas usage (86%) (Figure 2).20,23 Therefore, pMDI inhalers account for a very small proportion (≤0.1%) of global emissions.20,23
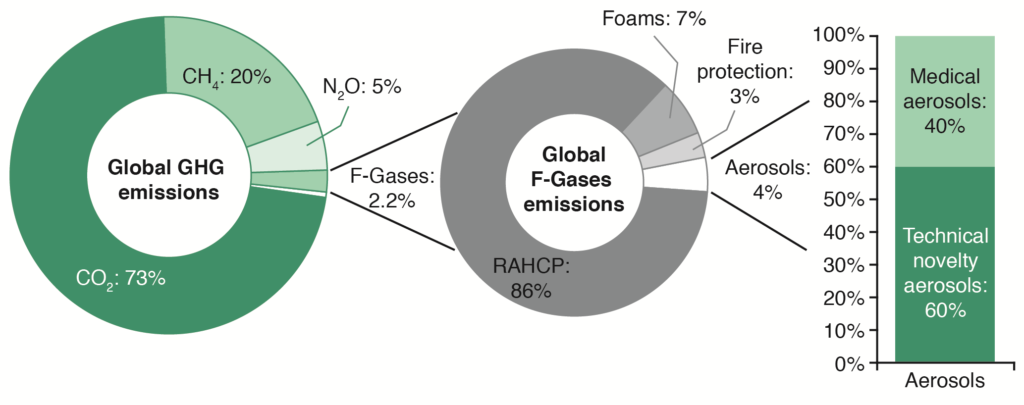
Figure 2: Global annual anthropogenic greenhouse gas (GHG) emissions by gas type and distribution of market use of fluorinated gases (F-Gases).20,23 (RACHP = refrigeration, air conditioning and heat pump.)
Overall, DPIs have a lower carbon footprint (CF) than pMDIs. Usage is the major CF contributor for pMDIs due to the presence of propellants.24,25 Conversely, for DPIs, raw materials for manufacturing are the greatest contributors.24,25 However, introducing a propellant with lower GWP could significantly reduce the CF of pMDIs to within the range of DPIs.24-26
POTENTIAL PATIENT DRAWBACKS
Many expert respiratory healthcare providers have expressed concern that implementation of a device switch initiative may lead to detrimental effects on quality of care and patient outcomes. Adverse outcomes have been previously demonstrated following an enforced switch of stable respiratory patients to alternative inhalers; switching resulted in reduced disease control and an increased number of healthcare visits in both asthma and COPD patients.27-29
The optimal choice for the most suitable inhaler for each patient is a complex decision taken between the treating physician and the patient.2,30 Patient preference and empowerment, through informed decision making, are vital to achieving the best possible outcomes in patients with COPD or asthma. If access to pMDIs is restricted, the physician’s ability to tailor treatment to patients will be limited. Moreover, many respiratory physicians caution that the implementation of a device switch initiative may create a stigma associated with the use of pMDIs, as emotive issues such as climate change may cause patients to feel pressurised into switching from their preferred therapy.
Switching from a patient’s preferred therapeutic option may be detrimental to their treatment outcomes; patients should not be stigmatised for taking approved medication that is essential for treating their condition. In asthma, a major challenge is to motivate people to take the correct treatment regularly, while in people with COPD, feelings of self-blame are common. Such stigmas may lead to decreased adherence to therapy, resulting in adverse effects on patient outcomes and their quality of life.31,32 Evoking feelings of guilt in those who need or choose pMDIs must be avoided; discussions on the issue of climate change need to be framed within the context of the wider political setting if the wider climate change issue is to be addressed meaningfully.
PATIENT-TAILORED INHALER CHOICE
Long-term disease control and patient management in asthma and COPD patients with low adherence remains a challenge. Poor inhaler technique remains a major barrier to achieving disease control in patients with asthma or COPD and is prevalent in 31% of patients.33 The importance of achieving correct delivery of drugs by efficient inhaler use cannot be underestimated; proper inhaler technique leads to improved symptom relief and quality of life – and reduces morbidity, mortality and acute hospital care costs.34-38 However, only 22% of patients have complete confidence in their inhaler technique.39
“Since pMDIs need to remain an option for patients, a major unmet need exists for low GWP alternative propellants in pMDIs.”
Many developments have been made to improve patient confidence and their inhaler technique, and hence improve patient outcomes. For example, most studies that have implemented inhaler technique educational programmes in patients with asthma or COPD have resulted in significantly improved inhaler technique following intervention.40 Stable patients with asthma whose treatment is initiated on pMDIs have achieved better disease control than those given the same drug prescribed with a DPI.41 Overall, evidence suggests that tailoring inhaler choice to a patient’s ability to use specific devices, coupled with ongoing education to support optimal inhaler usage, may improve patient confidence and enhance both adherence and disease control.42,43 Improved inhaler technique, adherence and disease control, in addition to proper disposal of empty inhalers, will contribute to reducing the total CF of pMDIs.22 Therefore, asking stable patients using pMDIs to switch to DPIs for non-clinical reasons is concerning, and will likely negatively impact disease control. Inhaler choice should be based on patient characteristics (Figure 3)42 and patient preference. Since pMDIs need to remain an option for patients, a major unmet need exists for low GWP alternative propellants in pMDIs to achieve a reduced CF in respiratory treatments, without risking adverse effects on patient outcomes.

Figure 3: Inhaler type decision tree in patients with asthma or COPD.42 (BA = breath actuated)
DEVELOPMENT OF A CARBON MINIMAL pMDI
Development of pMDIs containing a low GWP propellant have the potential to reduce the carbon footprint of pMDIs by 90%,25 but, critically, will also ensure a continued choice for physicians and patients, and avoid any negative impact on patient health. Recently, companies producing pMDI maintenance therapies have announced plans to introduce carbon minimal pMDIs by 2025.43,44 Chiesi’s planned carbon minimal pMDI uses Koura’s HFA 152a (1, 1-difluoroethane) as a candidate propellant.43
HFA 152a is classified as a low GWP propellant, as its GWP value is significantly lower (138 GWP for 100-year time horizon) than that of both HFC 134a and HFA 227ea (1,300 and 3,350 GWP for 100-year time horizon, respectively).23 Due to the lower liquid density of HFA 152a compared with HFA 134a and HFA 227ea, early indications are that a lower weight of propellant is needed per dose, resulting in additional carbon savings.10
Initial research into HFA 152a use in pMDIs has been promising, showing similar performance levels to HFA 134a and HFA 227ea.10 Given HFA 152a is used more commonly in consumer aerosols, the toxicology of HFA 152a is well characterised and is similar to HFA 134a.10 Studies to address the gaps in industrial toxicity knowledge have been successful; inhalation safety studies are underway and long-term toxicology testing on HFA 152a is expected to be completed in 2021.10 Moreover, first-in-human clinical trials have now begun.45

Figure 4: Comparison of the carbon footprint of three different types of inhaler.24
Development of HFA 152a inhalers will significantly reduce the CF of pMDIs, to a level within the range of DPIs (Figure 4).24 Short-term approaches to reduce the environmental impact by limiting use of pMDIs are likely to undermine innovation of such carbon-minimal pMDIs. Introducing a carbon-minimal pMDI will allow a seamless transition from pMDIs, providing large carbon savings but also maintaining patient choice and ensuring continuity of care without potential adverse health effects.
CONCLUSIONS
While the impact of harmful gases on our environment needs to be reduced, it is vital that any action taken does not inadvertently jeopardise patient safety and outcomes. Therefore, pMDIs must remain a prescription option for all asthma and COPD patients, particularly for those where pMDIs are the preferred choice. Chiesi’s plan – which includes the development of and the transition to pMDIs containing low GWP propellant (HFA 152a) – has the potential to offer environmental benefits whilst maintaining patient choice and well being.
REFERENCES
- GBD 2015 Chronic Respiratory Disease Collaborators, “Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015”. Lancet Respir Med, 2017, Vol 5(9), pp 691–706.
- Global Initiative for Asthma (GINA): Global strategy for asthma management and prevention. Update 2014 and Online Appendix. Available at http://www.ginasthma.org. Accessed 23 March 2020.
- World Health Organization (WHO), “Chronic obstructive pulmonary disease (COPD)”. 2019.
- Diab N, Gershon AS, Sin DD et al, “Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease”. Am J Respir Crit Care Med, 2018, Vol 198(9), pp 1130–1139.
- Lozano R, Naghavi M, Foreman K et al, “Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010”. Lancet, 2012, Vol 380(9859), pp 2095–128.
- Quaderi SA, Hurst JR, “The unmet global burden of COPD”. Glob Health Epidemiol Genom, 2018, Vol 3, e4.
- Usmani OS, “Choosing the right inhaler for your asthma or COPD patient”. Ther Clin Risk Manag, 2019, Vol 15, pp 461–72.
- Lavorini F, Corrigan CJ, Barnes PJ et al, “Retail sales of inhalation devices in European countries: so much for a global policy”. Respir Med, 2011, Vol 105(7), pp 1099–1103.
- Ferguson GT, Hickey AJ, Dwivedi S, “Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases”. Respir Med, 2018, Vol 134, pp 16–23.
- United Nations Environment Programme. Report of the Medical Technical Options Committee (MTOC) 2018 Assessment Report. 2018. Available at http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MTOC/MTOC-Assessment-Report-2014.pdf. Accessed 23 March 2020.
- The Montreal Protocol on substances that deplete the ozone layer. Final Act (Nairobi: UNEP1987). Federal Register 1994, 59FR56276–56298.
- United Nations Environment Programme. Report of the Medical Technical Options Committee (MTOC) 2014 Assessment Report. 2014. Available at http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MTOC/MTOC-Assessment-Report-2014.pdf. Accessed 23 March 2020.
- Carbon Footprint Italy 2019. Available: http://www.carbonfootprintitaly.it/p-2019-0008/ Accessed 23 March 2020.
- Nicolini G, Scichilone N, Bizzi A, “Beclomethasone/formoterol fixed combination for the management of asthma: patient considerations”. Ther Clin Risk Manag, 2008, Vol 4(5), pp 855–64.
- Leach CL, “The CFC to HFA transition and its impact on pulmonary drug development”. RespirCare, 2005, Vol 50(9), pp 1201–6.
- Crisafulli E, Pisi R, Ajello M et al, “Prevalence of Small-Airway Dysfunction among COPD Patients with Different GOLD Stages and Its Role in the Impact of Disease”. Respiration, 2017, Vol 93(1), pp 32–41.
- Postma DS, Brightling C, Baldi S et al, “Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study”. Lancet Respir Med, 2019, Vol 7(5), pp 402–416.
- Bousquet J, Poli G, Acerbi D, “Systemic exposure and implications for lung deposition with an extra-fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination”. Clin Pharmacokinet, 2009, Vol 48(6), pp 347–58.
- European Commission. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006 Text with EEA Relevance. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.150.01.0195.01.ENG Accessed: 23 March 2020.
- OzonAction Kigali Fact Sheet 2. 2017. Available at: https://wedocs.unep.org/bitstream/handle/20.500.11822/26867/7877FS02_C_Uses_EN.pdf?sequence=1&isAllowed=y Accessed 23 March 2020.
- House of Commons Environmental Audit Committee. UK Progress on Reducing F-gas Emissions. 18 April, 2018. Available at: https://publications.parliament.uk/pa/cm201719/cmselect/cmenvaud/469/469.pdf. Date last accessed: 23 March 2020.
- Wilkinson AJK, Braggins R, Steinbach I, Smith J, “Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England”. BMJ Open, 2019, Vol 9, e028763.
- IPCC Fifth Assessment Report (AR5), 2014. Available at: https://www.ipcc.ch/report/ar5/syr/ Accessed 23 March 2020.
- Jeswani HK, Corr S and Azapagic A, “Reducing carbon footprint of metered dose inhalers”. Inhalation, 2017.
- Jeswani HK, Azapagic A, “Life cycle environmental impacts of inhalers”. Journal of Cleaner Production, 2019, Vol 237, 117733.
- Janson C, Henderson R, Löfdahl M et al, “Carbon footprint impact of the choice of inhalers for asthma and COPD”. Thorax, 2020, Vol 75(1), pp 82–84.
- Björnsdóttir US, Gizurarson S, Sabale U, “Potential negative consequences of non-consented switch of inhaled medications and devices in asthma patietns”. Int J Clin Pract, 2013, Vol 67 (9), pp904–10.
- Björnsdóttir US, Sigurðardóttir ST, Jonsson JS et al, “Impact of changes to reimbursement of fixed combinations of inhaled corticosteroids and long-acting β2-agonists in obstructive lung diseases: a population-based, observational study”. Int J Clin Pract, 2014, Vol 68(7), pp 812–819.
- Doyle S, Lloyd A, Williams A, “What happens to patients who have their asthma device switched without their consent?” Prim Care Respir J, 2010, Vol 19(2), pp 131–9.
- Dolovich MB, Ahrens RC, Hess DR et al, “Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology”. Chest, 2005, Vol 127(1), pp 335–71.
- Lycett H, Wildman E, Raebel EM et al, “Treatment perceptions in patients with asthma: Synthesis of factors influencing adherence”. Respir Med, 2018, Vol 141, pp 180–9.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K, “Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD”. Respir Med, 2013, Vol 107(10), pp 1481–90.
- Sanchis J, Gich I, Pedersen S, “Systematic review of errors in inhaler use: has patient technique improved over time?” Chest, 2016, Vol 150(2), pp 394–406.
- Usmani OS, Scullion J, Keeley D, “Our Planet or our patients – is the sky the limit for inhaler choice?” Lancet Respir Med, 2018, Vol 7(1), pp 11–13.
- Giraud V, Allaert FA. “Improved asthma control with breath-actuated pressurized metered dose inhaler (pMDI): The SYSTER survey.” Eur Rev Medand Pharmaco, 2009, Vol 13(5), pp 323–30.
- Giraud V, Roche N, “Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability”. Eur Respir J, 2002, Vol 19(2), pp 246-51.
- Melani AS, Bonavia M, Cilenti V et al, “Inhaler mishandling remains common in real life and is associated with reduced disease control”. Respir Med, 2011, Vol 105(6), pp 930-8.
- Melani AS, Paleari D, “Maintaining Control of Chronic Obstructive Airway Disease: Adherence to Inhaled Therapy and Risks and Benefits of Switching Devices”. COPD: J Chron Obst Pulmon Dis, 2016, Vol 13(2), pp 241–50.
- Amin AN, Vaidyanathan G, Roughley A, Small M, “Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease”. Patient Prefer Adherence, 2017, Vol 11, pp 1205–12.
- Klijn SL, Hilgsmann M, Evers SM et al, “Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review”. NPJ Prim Care Respir Med, 2017, Vol 27(1), pp 24.
- Price D, Roche N, Christian Virchow J et al, “Device type and real-world effectiveness of asthma combination therapy: an observational study”. Respir Med, 2011, Vol 105(10), pp 1457–66.
- Dekhuijzen PNR, Vincken W, Virchow JC et al, “Prescription of inhalers in asthma and COPD: Towards a rational, rapid and effective approach”. Respir Med, 2013, Vol 107(12), pp 1817–21.
- “Chiesi outlines €350 million investment and announces first carbon minimal pressurised Metered Dose Inhaler (pMDI) for Asthma and COPD”. Press Release, Chiesi, December 4, 2019.
- “Investing in a sustainable future for patients with respiratory disease”. Press Release, AstraZeneca, January 22, 2020.
- “‘Green’ Medical Propellant Receives FDA Approval to Proceed to Clinical Trials”. Press Release, Koura, February 4, 2020.

