To Issue 161
Citation: Theobald N, “Are Oral Formulations of Oligonucleotide Therapeutics Within Reach?”. ONdrugDelivery, Issue 161 (May/Jun 2024), pp 3–5.
Nigel Theobald, discusses key issues and strategies for formulation scientists aiming to unlock the potential of oral delivery for small interfering ribonucleic acid drugs targeted against gastrointestinal diseases and cancers.
“For siRNA therapeutics in particular, there is a high level of consensus that they hold significant promise as improved therapeutic options for certain conditions, and offer a new tool against hard-to-treat diseases.”
Oral delivery has long been the preferred route for administering medication. It is the most common and convenient dosage form for the majority of the world’s population – it can be easily self-administered and is non-invasive, resulting in increased treatment compliance and greater population coverage. Oral delivery has always been considered most suitable for small molecule drugs; in fact, 60% of small molecules, which make up to 90% of the total commercial drug products, are administered orally.1
Naturally, as various classes of biologic therapeutics have emerged in recent years, oral delivery has been investigated for administering these drugs too. For small interfering ribonucleic acid (siRNA) therapeutics in particular, there is a high level of consensus that they hold significant promise as improved therapeutic options for certain conditions, and offer a new tool against hard-to-treat diseases, due to their ability to selectively downregulate or silence disease-causing genes.
However, siRNA therapeutics require novel delivery systems to achieve their clinical effectiveness, as they are susceptible to rapid elimination by gastrointestinal (GI) processes if administered without protection and are poorly absorbed into systemic circulation. So far, the six siRNA drugs approved by regulators are injectables and there are, as of yet, no large-scale clinical trials for any other dosage forms.2
TARGETING THE GI TRACT
In recent years, significant advancements have been made in understanding the genetic roots of many diseases and how undesirable gene expression can be downregulated, augmented or corrected with therapeutic success.
In particular, the specific advantages of siRNA therapeutics over alternative small molecule and monoclonal antibody (mAb) drugs that have been demonstrated are:3
- Simplicity: siRNA executes its function by complete Watson–Crick base pairing with messenger RNA (mRNA), whereas small molecule and mAb drugs need to recognise the complicated spatial conformation of proteins.
- Specificity: siRNA has a more distinct, specific mechanism of action with reduced side effects.
- Broad application: Theoretically, any gene of interest can be targeted by siRNA – only the correct nucleotide sequence is needed to match the targeted mRNA.
- Rapid development: High-throughput screening methods can identify effective siRNA sequences against disease targets, accelerating the drug development process, which means that siRNA is potentially faster to market over a wider therapeutic area.
“Well-known factors, such as pH, enzymatic activity and the surface area and structure of the intestine wall, dictate the extent and rate of drug absorption, making it essential for drug developers to tailor formulations accordingly.”
In addition, the complexities of delivering drugs via the GI tract have been extensively studied. From the mouth to the lower intestines and colon, it is clear that each segment presents formidable barriers and various absorption mechanisms that influence drug bioavailability. Well-known factors, such as pH, enzymatic activity and the surface area and structure of the intestine wall, dictate the extent and rate of drug absorption, making it essential for drug developers to tailor formulations accordingly, driving the development of novel strategies to enhance efficacy.
In parallel, dissatisfaction with current therapies for immune-mediated GI inflammatory diseases, such as inflammatory bowel disease (IBD) and Crohn’s disease, have stimulated research into siRNA. It holds promise as a targeted, precise therapy that could be used to treat intestinal diseases associated with the upregulation of specific proteins found in, for example, gut epithelial cells.
Common current treatments for IBD include amino salicylates, corticosteroids and immunosuppressives – interventions that often cause side effects such as hypertension, osteoporosis, depression and increased susceptibility to infections. These side effects are due, in large part, to the non-specificity of drug action and could be mitigated by the use of siRNA therapeutics, whether as a stand-alone or concurrent therapy. GI cancers, such as colorectal cancer, are also being targeted for new treatment approaches using siRNA.
UNDERSTANDING THE CHALLENGES
Looking to the current literature for guidance, it is striking to note that, in all reports on oral siRNA, there is a strong consensus around the common challenges over and above those faced for small molecules. Furthermore, there is a wide range of innovative formulation strategies being explored in an effort to overcome these key issues. These include controlled release formulations, enteric-coated lipid-based nanoparticles (LNPs) and polymeric carriers.4
A recent review article5 highlights the preclinical data for IBD and colorectal cancer, presenting a clear assessment of some of the barriers to oral delivery. Another group hypothesises that oral administration facilitates the direct delivery of siRNA to lesions within the small intestines and colon, making it the ideal approach for treating patients with IBD.5 In addition, scientists from the University of Texas6 and, separately, a team from a consortium of European researchers,5 completed comprehensive reviews that summarise the formulation challenges and delivery strategies for oral delivery of siRNA, reporting on recent advancements from basic research towards the preclinical stage of drug development.
In summary, their findings indicate that, in order to translate oral formulations of siRNA from research in animal models to human clinical trials for specific diseases, drug developers must overcome a threefold challenge:
- Protect the siRNA as it travels through the GI tract
- Develop a formulation to deliver the siRNA to the targeted part of the GI tract and the correct cell type (e.g. macrophage)
- Selectively release the siRNA at the site of action.
NOVEL ORAL sIRNA DELIVERY SYSTEMS
A comprehensive exploration of all available options is beyond the scope of this article; however, the following is a sample of example strategies for oral siRNA delivery that are currently in preclinical research.
Lipid Nanoparticles
Given the well-established use of liposomes and LNPs in both small molecule and gene therapy applications, the first approved siRNA drugs have been intravenous (IV) formulations using LNPs. As such, a number of researchers have used LNPs as a starting point for oral siRNA delivery studies. However, LNPs are not without significant challenges; the literature describes a wide range of critical factors for successful development, namely:
- Particle size and formulation options
- Compatibility and safety
- Protection and targeting
- Uptake and efficacy
- Metabolism and clearance
- Cost and scale-up potential.
One study, conducted by a group experienced with LNP formulation,7 concentrated on efficacy and metabolism, using a combination of in vitro and mouse models to better understand the fate of LNPs in the GI tract. They studied LNP delivery under deconstructed stomach and intestinal conditions to assess stability over the expected pH range found in the GI tract, as well as looking at biodistribution and potency in mouse studies.
In summary, the authors noted that the LNP formulation was stable under varying pH levels and that LNPs entered the cells of the small intestine and colon and remained in the gut for up to eight hours following administration. They also saw, however, that enzyme degradation significantly reduced LNP efficacy and prevented gene-silencing activity in vivo. They suggest that orally delivered LNPs need to be protected in the stomach and upper intestine in order to achieve siRNA delivery to intestinal epithelial cells.
Milk-Derived Exosomes
In recent work, a group from the Korea Institute of Science and Technology (KIST) in Seoul investigated oral tumour necrosis factor alpha (TNF-α) siRNA delivery via milk-derived exosomes (M-Exos) for effective treatment of IBD.8 The study highlighted that M-Exos offer superior structural stability compared with other LNPs and that, in the group’s experiments, they were an efficient siRNA carrier.
M-Exos were loaded with TNF-α siRNA and efficacy in treating colitis was assessed in a dextran sulphate sodium (DSS)-induced inflammatory bowel disease murine model. The results demonstrate that M-Exos loaded with TNF-α siRNA effectively inhibited the expression of TNF-α-related inflammatory cytokines. Moreover, given that M-Exos are composed of unique lipids with high bioavailability, orally administered M-Exo/siRNA effectively reached colonic tissues, leading to decreased TNF-α expression and successful alleviation of colitis symptoms.
Engineered Silica Nanoparticles
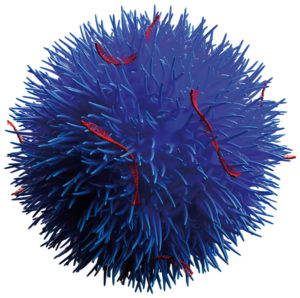
Figure 1: Visualisation of a Nuvec particle.
Mesoporous silica nanoparticles (MSNs) have been identified as a suitable route for drug delivery due to their size, relatively inert nature and extremely large specific surfaces that can be functionalised by therapeutic and targeting entities. A series of preclinical studies using Nuvec®, a novel silica nanoparticle (Figure 1), have shown that the surface structure provides the siRNA with protection from enzyme attack as the siRNA cargo is held within the spikes on the particles.
With regard to oral delivery, further processing can provide a lyophilised, powdered form that allows loading of the siRNA/Nuvec intermediate into enteric-protected capsules (and potentially to be compressed into tablets) for targeting particular GI locations. At the site of action, the particle enters the target cell where the siRNAs detect and degrade a homologous mRNA sequence in the cell, resulting in reduction of the relevant protein and consequent inhibition of cell growth. This mode of action allows for precise targeting and the inhibition of identified signalling pathways with reduced toxicity, immune system response and potential side effects.
Experiments have confirmed the successful in vivo oral administration of Nuvec® loaded with a deoxyribonucleic acid (DNA) plasmid for ovalbumin. The therapeutic was administered by enteric-coated capsule and the contents, having been released in the intestinal lumen, were taken up by intestinal cells, with successful transfection and release of the newly synthesised ovalbumin. In a study conducted earlier this year, an enterically coated capsule containing Nuvec loaded with a DNA plasmid for ovalbumin was administered to mice and both protein/antigen and immunoglobulin G (IgG) antibody expression was observed. This research is ongoing.
LOOKING AHEAD
Moving through 2024, the increased research activity around siRNA looks set to deliver on the long-heralded potential for targeted gene therapy. Importantly, oral drug delivery remains the preferred avenue for siRNA administration, offering convenience and patient compliance, especially for long-term conditions such as IBD and Crohn’s disease. This places the work of formulation scientists at the leading edge of drug development and, whilst challenges persist, ongoing research efforts and technological innovations are driving the field forward, opening new possibilities for improved drug efficacy and patient outcomes.
By harnessing the power of innovative formulation strategies and advanced drug delivery technologies, many believe the potential of oral oligonucleotide drug delivery can be unlocked. This is arguably the most cost-effective route for drug developers due to lower sterility constraints, more flexibility in design of dosage form and ease of production, compared with current IV formulations.
REFERENCES
- Kumari N et al, “Oral Delivery of Nucleic Acid Therapies for Local and Systemic Action”. Pharm Res, 2023, Vol 40(1), pp 107–122.
- Sehgal I, Eells K, Hudson I, “Oral Delivery of Nucleic Acid Therapies for Local and Systemic Action”. Pharmacy (Basel), 2024, Vol 12(2), Article 58.
- Hu B et al, “Therapeutic siRNA: state of the art”. Signal Transduct Target Ther, 2020, Vol 5(1), Article 101.
- Lou J et al, “Advances in Oral Drug Delivery Systems: Challenges and Opportunities”. Pharmaceutics, Vol 15(2), Article 484.
- O’Driscoll CM et al, “Oral delivery of non-viral nucleic acid-based therapeutics – do we have the guts for this?”. Eur J Pharm Sci, 2019, vol 133, pp 190–204.
- Afrin H et al, “Oral delivery of RNAi for cancer therapy”. Cancer Metastasis Rev, 2023, Vol 42(3), pp 699–724.
- Ball RL, Bajaj P, Whitehead KA, “Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract”. Sci Rep, 2018, Vol 8(1), Article 2178.
- Han G et al, “Oral TNF-α siRNA delivery via milk-derived exosomes for effective treatment of inflammatory bowel disease”. Bioact Mater, 2023, Vol 34, pp 138–149.

