To Issue 152
Citation: Thompson I, “Autoinjectors for Large-Volume Subcutaneous Drug Delivery”. ONdrugDelivery, Issue 152 (Oct 2023), pp 26–31.
Ian Thompson provides a summary of a recent literature review1 on subcutaneous administration with dose volumes greater than 1.0 mL, which examines how previous studies have addressed the limitations and considerations for designing and developing large-volume autoinjectors.
EMERGENCE OF LARGE-VOLUME INJECTIONS
Subcutaneous drug delivery has emerged as a viable, and often preferred, alternative to intravenous infusion of biologics, offering patients and healthcare providers new home-based treatment options that improve treatment adherence, reduce the cost of therapy and decrease the burden on healthcare resources. Although subcutaneous delivery options advance patient-centric care, they face numerous development challenges, ranging from pharmacokinetics and efficacy to bioavailability, viscosity, stability and designing delivery devices that are safe and effective for home use.
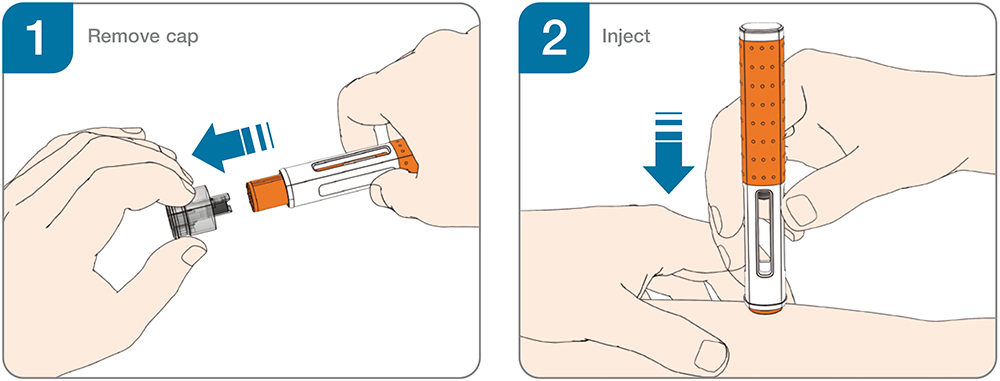
Figure 1: Two-step handling for autoinjectors.
“The review concluded that the literature supports the feasibility of delivering single large-dose subcutaneous volumes, providing a foundation for more widespread use of large-volume autoinjectors.”
The availability of user-tested device platforms has broadened access to handheld autoinjectors for biologics in chronic diseases such as rheumatoid arthritis, cardiovascular diseases, obesity, asthma, migraine, psoriasis, rare diseases and immuno-oncology (Table 1). Simple two-step handheld autoinjectors (Figure 1) have become a preferred option for the safe and effective self-administration of single doses and have been widely adopted as the industry standard for subcutaneous drug delivery.
| Product | Manufacturer | Presentation |
| AJOVY (fremanezumab) | Teva Pharma | AI, PFS |
| COSENTYX (secukinumab) | Novartis Pharma | AI, NSD |
| DUPIXENT (dupilumab) | Sanofi/Regeneron | AI, NSD |
| LEQVIO (inclisiran) | Novartis Pharma | PFS |
| PRALUENT (alirocumab) | Sanofi/Regeneron | AI |
| SILIQ (brodalumab) | Valeant Pharmaceuticals | PFS |
| TEGSEDI (inotersen) | Akcea/Ionis | PFS |
| TEZSPIRE (tezepelumab) | AstraZeneca/Amgen | AI, NSD |
| TAKHZYRO (lanadelumab) | Takeda Pharma | PFS |
| WAYLIVRA (volanesorsen) | Akcea/Ionis | PFS |
Table 1: Approved products from the US FDA and EMA using 2.25 mL PFSs (prefilled syringes), NSDs (needle shield devices) and prefilled handheld autoinjectors (AIs).
For a long time, the pharma industry has assumed the delivery of 1.0 mL in less than 10–15 seconds to be the feasible upper limit for handheld autoinjectors. However, since 2020, approvals of products with single-dose volumes up to 2.0 mL have demonstrated the successful delivery of larger volumes.
These recent advances have energised attempts to expand the feasible volume limit for handheld autoinjectors. Higher injection volumes not only reduce the required frequency of injections, prove preferable to patients and enhance therapy adherence, but also help establish subcutaneous dosing for new therapeutic areas and drug modalities that require larger single-dose volumes. As such, the advent of handheld autoinjectors exceeding 2.0 mL capacity has garnered significant attention.
Most recently, Ypsomed has introduced the YpsoMate 5.5 (Figure 2) autoinjector for 5.5 mL prefilled glass syringes triggered by push-on-skin activation based on a new ready-to-use 5.5 mL staked-needle prefilled syringe format. Only further clinical studies will determine what upper limits of injection volumes can be achieved practically with high-volume handheld autoinjectors.

Figure 2: YpsoMate 5.5 large volume two-step autoinjector taking handheld self-injection beyond volumes of 2.0 mL.
LITERATURE REVIEW OF LARGE-VOLUME SUBCUTANEOUS INJECTIONS
Ypsomed recently published a review evaluating the literature on subcutaneous administration with dose volumes greater than 1.0 mL.1 The review concluded that the literature supports the feasibility of delivering single large-dose subcutaneous volumes, providing a foundation for more widespread use of large-volume autoinjectors. It covered 31 studies on large-volume subcutaneous delivery and structured findings based on three aspects critical to developing large-volume autoinjectors:
- Injection tolerability, as larger injection volumes, which may intensify injection-site reactions, change the subcutaneous depot location and increase pain
- Suitability for self-administration, as manufacturers must ensure safe and effective drug self-administration as autoinjectors help shift the point of care from the hospital to the home
- Pharmacokinetic equivalence with existing dosing options tested in multicentre pivotal studies with safety and efficacy end points in subsequent bridging studies.
The review analysed prior research on large-volume subcutaneous injections across therapeutic contexts and device categories, such as manual syringes, syringe pumps, wearable large-volume injectors and handheld autoinjectors. While these studies have advanced understanding of large-volume subcutaneous injections in general, they do not address certain issues specific to high-rate and large-volume injections with handheld devices. Therefore, this review suggests avenues for future work on large-volume autoinjectors within the three aspects previously described (Table 2).
| Future Research Topic | Injection Tolerability | Suitability for Self-Administration | Pharmacokinetic Equivalence |
| Injection tolerability |
• Effects of device technical attributes (e.g. needle guard geometry) on injection tolerability • User-related force and its relationship with pain and injection-site leakage (e.g. force required to trigger injection) • Relative importance of injection volume and rate versus other drug-specific parameters (e.g. formulation) for injection tolerance • Pain related to large-volume autoinjectors versus other device categories (e.g. syringe pumps and large-volume wearable devices). |
• What injection duration keeps pain low while ensuring safe and effective device use |
• Injection tolerability/pain-related end points in pharmacokinetic bridging studies. |
| Suitability for self-administration |
– |
• Optimising user experience and interface for prolonged injection duration (e.g. continuous injection feedback) • User preferences for different autoinjector-based dosing options (e.g. injection duration versus frequency) • User preferences across large-volume device alternatives (e.g. manual syringes, handheld autoinjectors, syringe pumps, large-volume wearable devices). |
• Virtual at-home clinical trials to include usability-related end points and self-assessment on patient preference. |
| Pharmacokinetic equivalence |
– | – |
• Impact of formulation advances that enable rapid injection of high-concentration biologics on pharmacokinetic profiles • Validation of robustness of pharmacokinetic profile against changes in injection frequency • Innovative approaches to molecule-independent pharmacokinetic bridging to simplify access to large-volume autoinjectors. |
Table 2: Proposed avenues for future work on large-volume autoinjectors covering injection tolerability, suitability for self-administration and pharmacokinetic equivalence.
“Future work should examine how user preferences for large-volume autoinjector-based dosing options change with differences in dosing regimens.”
These themes are not only helpful in organising the existing literature but also provide the basis for categorising future research. First, the results of the review call for future studies within the three themes that address questions related to high-rate injections with high-volume autoinjectors. Second, they highlight the need for integrative work that spans the three themes. Advancing topics at the intersection of these themes will promote comprehensive views of the feasibility of large-volume autoinjectors and help to address critical trade-offs in their design and development.
Injection Tolerability
The review advances the key insights that, although higher injection volumes and rates may increase pain, the impact was low on the pain scale and that drug formulation may mask these effects. These findings will inform future work on handheld autoinjectors for high-volume dosing. Effective subcutaneous dosing with handheld autoinjectors will likely hinge on new formulations that allow rapid absorption of highly concentrated biologics in subcutaneous tissue; for example, the co-formulation of the dispersion enhancer hyaluronidase has effectively improved subcutaneous delivery.
Still, new formulations may also affect pain perception. Pain is particularly significant in the case of large-volume autoinjectors, as users may remove the device prematurely from the injection site during prolonged injection times if it is too painful. Future work should therefore study the impact of such formulation advances on pain-related clinical outcomes.
Suitability for Self-Administration
The review presents strong evidence of the feasibility of self-administrating single large-volume doses. Researchers have demonstrated safe and effective use for handheld autoinjectors up to 2.25 mL, wearable large-volume injectors and large-volume manual syringe and infusion pumps. Studies show that increasing injection volume and time was feasible for handheld autoinjectors.
However, the review shows conflicting views on user preferences for different dosing options. Therefore, future work should examine how user preferences for large-volume autoinjector-based dosing options change with differences in dosing regimens. Previous studies have shown that injection duration and frequency play a significant role in treatment choices and adherence.
The review also calls for future work on user preferences across device categories, such as manual syringes, handheld autoinjectors, wearable large-volume injectors and syringe pumps. As the variety of devices continues to increase, research must provide healthcare professionals and patients with the necessary evidence to make treatment decisions for optimal adherence and therapy outcomes.
Pharmacokinetic Equivalence
The review found the pharmacokinetic profiles to be stable in response to changes in injection parameters. These findings are in line with the slow absorption rate of therapeutic proteins from the subcutaneous extracellular matrix. However, previous studies have focused mainly on switching from multiple small-volume injections to a single large-volume dose without adjusting the time intervals between injections. Future research should examine the effects of reducing injection frequency using large-volume autoinjectors on pharmacokinetic equivalence. Such a shift in dosing could potentially lead to improved treatment adherence and patient preference.
The review also highlights the potential for future work to evaluate novel approaches to assess the pharmacokinetic equivalence of high-volume autoinjectors compared with low-dose injections that are administered more frequently. Currently, new subcutaneous dosing options are established through dedicated drug-by-drug bridging studies. However, future work could consider molecule-independent approaches to clinical bridging studies to facilitate access to and accelerate the time-to-market of new large-volume handheld autoinjectors.
“The study of large-volume subcutaneous drug delivery has developed into a vibrant field of research, where researchers have made significant headway in exploring the upper limits of subcutaneous injection.”
A VIBRANT FIELD OF RESEARCH
Autoinjectors have effectively emerged as a viable option for safe and effective subcutaneous self-injection of up to 2.0 mL. Handheld devices for single doses exceeding 2.0 mL would be the next step in this incremental development. Devices under development leverage the well-accepted and proven push-on-skin handling principle, which may allow more seamless healthcare provider and patient onboarding with lower training requirements.
The study of large-volume subcutaneous drug delivery has developed into a vibrant field of research, where researchers have made significant headway in exploring the upper limits of subcutaneous injection. While researchers have yet to convince the industry, healthcare providers, patients and regulatory authorities of the feasibility of large-volume handheld autoinjectors for volumes between 2.0 and 5.0 mL, or even beyond, this review provides a valuable basis for developing these new dosing options. With the increasing demand for safe and effective self-administration options, large-volume autoinjectors could soon gain a foothold in the market.
COMPATIBILITY WITH EXISTING MANUFACTURING INFRASTRUCTURE
Novel large-volume autoinjectors may be able to leverage the potential of established technologies, manufacturing processes and regulatory pathways. These advantages could enable pharmaceutical companies to reinforce existing capabilities, mitigate risks in device development and accelerate time-to-market. For example, some large-volume autoinjector device platforms are largely compatible with existing infrastructure and manufacturing processes, such as fill-finish, final assembly and packaging.
Finally, large-volume handheld autoinjectors reduce injection duration, which has been shown to increase treatment preference and contribute to the widespread market acceptance of subcutaneous drug delivery. By allowing faster injection of large single-volume doses, large-volume autoinjectors may further boost the acceptance of subcutaneous injections.
While large-volume autoinjectors offer significant potential benefits, these devices also face barriers to adoption. Pharmaceutical manufacturers must establish new primary packaging suitable for high-volume drug delivery and address questions around high-concentration drug formulation, process development, analytical methods and drug stability. For investigational new drugs where time-to-market is critical, pharmaceutical manufacturers are more likely to adopt well-characterised syringes or cartridges for low-volume dosing systems and turn towards innovative large-volume dosing options only later in the lifecycle management process.
CONCLUSION
Large-volume handheld autoinjectors have the potential to offer new dosing regimens for drugs already injected subcutaneously and to expand subcutaneous injections to new fields, such as oncology. Therefore, these devices may become instrumental in broadening access to innovative medicines as they help shift the point of care from the hospital to the home. In oncology, for example, efforts are underway to establish more flexible care concepts where nurses can perform at-home injections and patients self-report symptoms during therapy.
The high-rate delivery of biologics with large-volume autoinjectors may further improve patient satisfaction, reduce healthcare resource use and increase advantageous effects on the total healthcare and societal costs of subcutaneous drug administration. However, patient preferences for devices and dosing regimens are complex and subject to change. Thus, we anticipate multiple dosing and delivery options to co-exist in the future, providing more flexibility to personalise treatment decisions to patients’ diverse needs. The potential benefits to patients and healthcare providers make handheld autoinjectors for the subcutaneous delivery of large-volumes a field worth exploring.
To learn more about Ypsomed’s drug delivery devices solutions, visit: yds.ypsomed.com.
REFERENCE
- Scheider A et al, “Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions”. Expert Opin Drug Deliv, 2023, Vol 20(6), pp 815–830.
Previous article
Interview with Claire Raynal-Olive & Pierre-Yves FavennecNext article
INTERVIEW with Jeff Henderson
