To Issue 149
Citation: Dobrev V, Müller P, Weiler R, “Real-World Evidence in the Age of Digital Health – Unlocking Value for Pharmaceutical Companies.” ONdrugDelivery, Issue 149 (Jun 2023), pp 39–42.
Ventsislav Dobrev, Philippe Müller and Rafael Weiler explore how real-world evidence in the age of digital health can help unlock value for pharmaceutical companies.
Digital health solutions have the potential to revolutionise the healthcare industry, providing opportunities for improved patient care, enhanced data collection and valuable real-world evidence (RWE). They enable the continuous monitoring of patients’ health and progress regarding their treatment.
“In recent years, technological capabilities have expanded, and the use of person-generated digital health data has increased in importance.”
Wearable devices and mobile applications can track various parameters, such as heart rate, sleep patterns, physical activity and medication adherence. By collecting real-world data (RWD), healthcare providers can gain a comprehensive understanding of patients’ health trajectories and make more informed clinical decisions. All these can contribute to personalised medicine, early detection of diseases and remote patient management – ultimately leading to improved patient outcomes.
In recent years, technological capabilities have expanded, and the use of person-generated digital health data has increased in importance. The recent trend for value-based and patient-centric care provides an opportunity for person-generated digital health data to play a fundamental role in the generation of RWE. RWE can be defined as “insights generated from RWD using appropriate scientific analytics with the intention to support a claim or belief, for which a hypothesis is usually formulated in advance”.
RWD, from which RWE is derived, can be defined as follows: “Longitudinal patient-level data captured in the routine management of patients (for care, cost management or public health) which can be repurposed to study the impact of healthcare interventions”.1 This article explores the opportunities that RWE derived from Ypsomed’s smart solutions can bring to pharmaceutical companies.
Digital health and RWE are linked as digital health facilitates the generation of RWE. RWE requirements and gaps demand data generation by new and innovative means. Despite the embrace in recent decades of evidence-based medicine, scientific evidence does not yet exist for the effectiveness of many clinical interventions, and the evidence that does exist is often surprisingly weak. Those two facts contribute to the so-called “evidence crisis”.
“New analytical tools for research are necessary for translating the rapidly accumulating quantities of standardised patient data into clinical guidelines.”
Two important enablers are necessary to overcome this crisis – common digital standards and open digital platforms. They need to be developed for the routine capture, sharing and analysis of health outcomes and other relevant data across health systems. New analytical tools for research are necessary for translating the rapidly accumulating quantities of standardised patient data into clinical guidelines for increasingly customised interventions, ever more precise care pathways and, ultimately, advanced decision-support tools to inform clinical practice and improve value for defined patient segments over time.2
YPSOMED SMART SOLUTIONS
Ypsomed smart solutions empower patients to improve health outcomes, connect stakeholders and support the generation of RWE (Figure 1). They represent an innovative digital ecosystem aimed at transforming therapy management for patients. The multicomponent system connects a patient-facing mobile app to manage therapy, a web portal and related cloud services for analytics and a connected injection device. The Ypsomed smart solutions platform provides a turnkey solution to continuously capture and analyse medication adherence data, drug and dosage data, patient-reported outcomes data and device data. As such, it collects a broad range of RWD, which can be leveraged by healthcare providers, regulators, insurance companies and pharmaceutical companies.
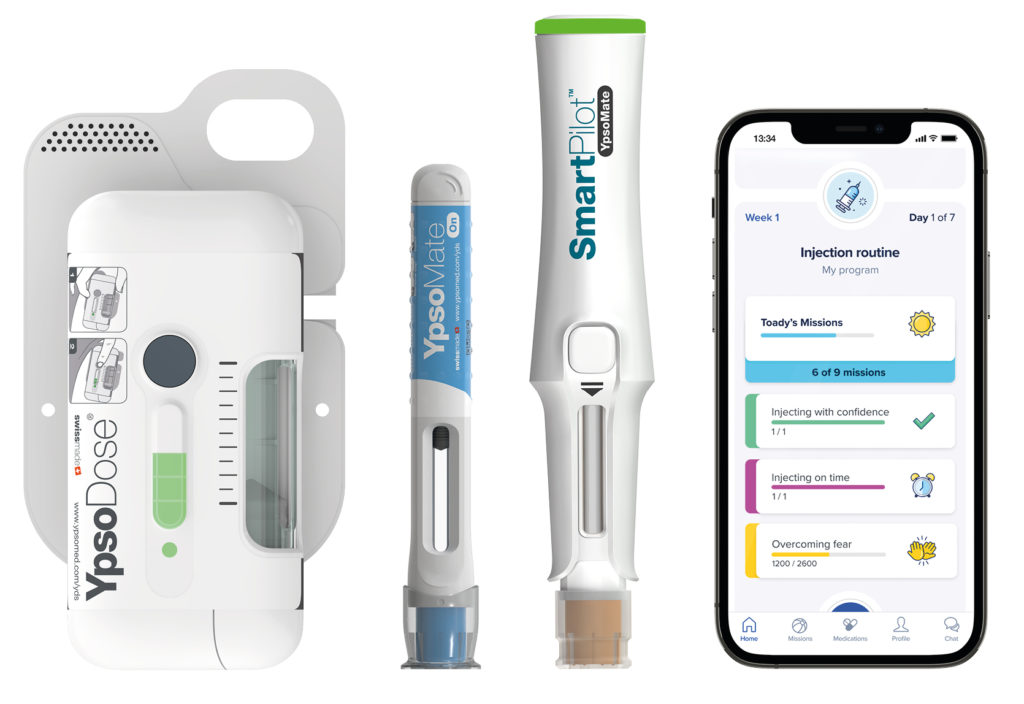
Figure 1: Ypsomed smart solutions.
To deliver innovative therapy management solutions, Ypsomed has partnered with leading digital health players such as Sidekick Health (Reykjavik, Iceland) and S3 Connected Health (Dublin, Ireland). By combining their extensive knowledge in self-injection therapies with best-in-class digital capabilities, the companies reduce complexity and offer an integrated device and digital solution. While the therapy management solutions support the patient all along the treatment cycle, they also generate RWE by capturing adherence and dosage data from the connected injection device and patient-reported outcomes (PROs) such as symptoms and mental health status.
These data points are then processed into meaningful and easily accessible information for patients – for example, summarised in an injection logbook to see taken and upcoming doses, as well as previously used injection sites. Further, the PRO data show the treatment progress over time by summarising data from symptom tracking, adherence, wellbeing, sleep and further vital signals.
“By leveraging RWE, pharmaceutical companies can enhance patient safety, improve treatment outcomes and make more informed decisions throughout the product lifecycle.”
However, these data points are not only relevant for patients but also provide important information about treatment effectiveness, adherence and drug safety for healthcare professionals and pharmaceutical companies. For this purpose, the Ypsomed smart solutions ecosystem includes a web dashboard for pharmaceutical companies with aggregated adherence and PRO data, which can be leveraged for post-market surveillance and research and development.
By leveraging smart solutions, Ypsomed aims to set an industry standard for embedding connected devices into digital ecosystems. Its solutions are compliant with relevant industry standards and certified for medical device quality system regulations, ensuring seamless integration, improved therapy management and, ultimately, better patient outcomes. At the same time, Ypsomed smart solutions support the generation of RWE and PROs, which can serve all stakeholders in the healthcare system for better value-based healthcare.
The Value of RWE for Pharmaceutical Companies
Many industry reports and consultancy companies argue about the potential of RWE. A white paper from IQVIA3 defines six areas that generate revenue growth and cost or productivity improvements throughout the product lifecycle. The white paper says the consensus is that RWE offers US$300–450 billion (£241–362 billion) in top-down opportunity for US healthcare alone. It says, at the same time, many life science companies are seeing ad hoc value from selected RWE case studies, often demonstrating as much as $100 million of bottom-up impact. The six value areas of RWE for pharmaceutical companies are as follows:
Improve Clinical Development, Clinical Trial Design and Patient Recruitment: By analysing RWD, pharmaceutical companies can gain insights into patient demographics, disease progression patterns and treatment use, which can aid in patient recruitment and trial planning. This can lead to more efficient and targeted clinical trials, reducing costs and time to market.
Better Market Access and Health Technology Assessments: RWE data is increasingly recognised and used by regulatory agencies and health technology assessment bodies. The result is that products may get to market faster.
Improving Launch Planning and Tracking: Digital health solutions can be used by pharmaceutical companies during the development and pre-launch phase, which allows the collection of the valuable insights needed for launch planning.
Optimising Commercial Spend Effectiveness: Pharmaceutical companies can improve their understanding of patient journeys and market dynamics through using digital health solutions that deliver patient insights and RWE. Marketing and sales departments of pharmaceutical companies can focus on specific points in the patient journey or physician’s decision-making process.
Demonstrating Ongoing Safety and Value: Safety and value demonstration studies are the historical domain of RWE, and they drive numerous decisions. This is not just about downside avoidance, but also driving increased product use up to the late stages of a brand’s lifecycle.
Increased Productivity and Cost Savings: RWE from digital health solutions may drive not only upside opportunities as outlined above but also significant advances in productivity and cost savings (Figure 2).
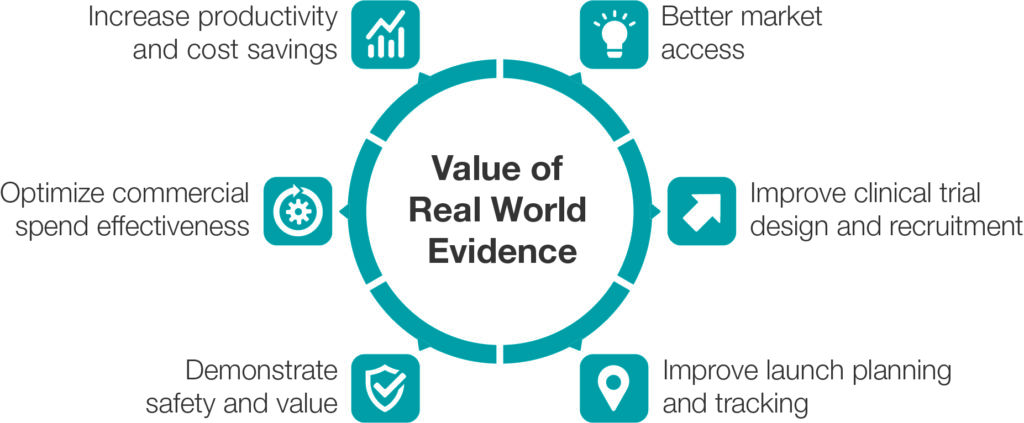
Figure 2: Value of RWE for pharmaceutical companies.
Case Study: Illustrating the Value from RWD/RWE Using Ypsomed Smart Solutions
A pharmaceutical company decides to use Ypsomed smart solutions for its cardiovascular drug in development. This allows it to collect precious patient insights such as:
Injection data: The connected autoinjector captures data related to the injection process, such as date, time, injection location, injection technique and factors that influence the proper injection practices. By tracking each injection versus dosing schemes, it allows monitoring and potentially influences therapy adherence.
Symptom tracking data: This feature of the therapy management app allows tracking of patient symptoms, disease flare-ups and changes in the disease condition, allowing timely intervention from the medical team.
PROs: The therapy management app can enable patients to complete PRO questionnaires or surveys that assess their quality of life, functional status or disease-specific outcomes. Collecting PRO data provides insights into treatment effectiveness and patient-reported benefits or limitations.
User feedback and preferences: The therapy management app can enable patients to provide feedback about their experience with the autoinjector, the quality of information delivered, etc. This feedback gives better understanding of the patient experience and helps improve it.
Other types of data agreed during the customisation phase: These can, for example, support different stakeholders involved in the therapy management cycle of the patient.
The pharmaceutical company uses Ypsomed smart solutions during its clinical trial phase, aiming to facilitate collecting longitudinal data from patients for the following purposes:
- Insights collected can guide the pharmaceutical company as to what kind of support to incorporate in its patient-support programmes at launch. This prevents investments in ineffective patient-support activities during the commercial phase.
- The pharmaceutical company can leverage the data to support the regulatory submission, demonstrate real-world effectiveness and safety, and provide additional evidence for the labelling claims. This approach accelerates market access and significantly improves the launch uptake curve.
- RWD and insights collected directly from patients reduce reliance on activities such as primary market research, which are often less scalable, more time consuming and less cost effective.
- Optimisation and customisation of Ypsomed smart solutions to be ready at drug launch to serve the target patient population in the best possible way.
The RWD and RWE delivered through Ypsomed smart solutions allows the pharmaceutical company to monitor the safety and efficacy of its product after it has been approved and launched in the market. By analysing the data, the pharmaceutical company could identify potential adverse events, drug interactions and long-term safety profiles. This information helps it proactively address any emerging safety concerns, make necessary product modifications and ensure patient safety.
CONCLUSION
Overall, RWE data from digital health solutions offers pharmaceutical companies a comprehensive understanding of how their products perform in real-world settings. This data can inform post-marketing surveillance, support market insights and commercialisation efforts, optimise clinical trial design, and facilitate regulatory submissions and health technology assessments. By leveraging RWE, pharmaceutical companies can enhance patient safety, improve treatment outcomes and make more informed decisions throughout the product lifecycle.
REFERENCE
- Khosla S et al, “The alignment of real-world evidence and digital health: realising the opportunity.” Ther Innovn Regul Sci, 2021, Vol 55(4), pp 889–898.
- Larsson S, Clawson J, Howard R, “Value-Based Health Care at an Inflection Point: A Global Agenda for the Next Decade.” NEJM Catalyst, Feb 24, 2023.
- Hughes B, Kessler M, McDonell A, “Breaking new ground with RWE: how some pharmacos are poised to realize a $1 billion opportunity.” White Paper, IQVIA, 2014.



