To Issue 164
Citation: “Product Showcase: CCBio’s Quick Dose On-Body Injector.” ONdrugDelivery, Issue 164 (Sep 2024), pp 34–35.
The vital role of patient self-administration of medication was highlighted during the lockdowns of the covid-19 pandemic. Conflicts around the world only add to the challenges of managing chronic conditions – creating drug shortages, for example, as well as difficult conditions for taking care of health needs. In addition, the characteristics of many new drugs mean that they often require specific drug containers.
All these factors mean that the concept of lifecycle management for medical devices is becoming a key topic in pharmaceutical resource management. Regulatory authorities are requiring drug delivery systems (DDS) to be reusable in multiple situations – increasing efficiency and reducing medical waste. How to increase the reuse of a DDS while being compatible with diverse drug containers has become an important issue.
Quick Dose is a revolutionary reusable on-body injector (OBI) designed and developed to address the challenges of drug container compatibility and extend the lifecycle of DDSs. The device aims to provide a more efficient and comfortable drug delivery method for patients and meet the evolving needs of the pharma and biotech industries, public health and even military personnel.
POWERFUL TECHNOLOGY FOR INJECTOR INNOVATION
Quick Dose was developed by CCBio, which has a strong technical background and has focused on developing DDSs for more than two decades. By integrating Taiwan’s electronic components and medical information resources, the company has achieved medical mechatronics capabilities. The new Quick Dose OBI is designed to address the challenges of subcutaneous (SC) drug delivery in many different scenarios.

Figure 1: The Quick Dose device with drug container cartridges.
Quick Dose consists of three components: a reusable infusion driver, a disposable injection device and a drug container (Figure 1). This design also aligns with the industry’s increasing interest in SC drug formulations and wearable OBI medical devices.
Quick Dose preserves the compatibility specifications of drug containers, allowing pharmaceutical companies to focus more on the therapeutic effectiveness of their drugs. Quick Dose can be applied to mainstream drug containers, including infusion bags, cartridges and prefilled syringes (Figure 2).
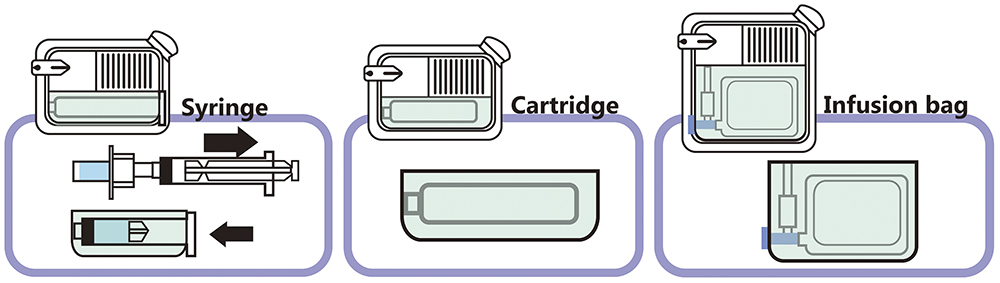
Figure 2: The Quick Dose device with mainstream drug container type.
Quick Dose also takes into account the necessary hygiene and safety factors. The needle set and drug solution circulation catheter are integrated into a disposable patch, ensuring that the item can be discarded after use. This design achieves a balance between safety and product integrity.
“The fully customisable programmability empowers patients to take control of their treatment, making their daily life easier and more comfortable.”

Figure 3: The Quick Dose’s reusable infusion driver.
Quick Dose has a reusable infusion driver (Figure 3). The fully customisable programmability empowers patients to take control of their treatment, making their daily life easier and more comfortable. The user interfaces of Quick Dose are well designed and technologically advanced, featuring options for WiFi, near field communication and Bluetooth connectivity.
The Quick Dose device has undergone successful preliminary design verification testing studies. CCBio used a standard 100 poise viscosity solution without any drug contents. The company tested mainstream drug containers of 21 mL infusion bags, 5 mL prefilled syringes and 3 mL cartridges. From the results, the Quick Dose device proved drug delivery capability to handle injection from a drug container within one hour.
METICULOUS EVALUATION FOR FORMATIVE USABILITY
From the perspective of both regulatory departments and pharmaceutical companies, OBI products are an emerging product category. Quick Dose focuses on usability formative evaluation. During the design phase and prior to formal tool development, a cognitive walkthrough approach was used to conduct formative evaluations of the Quick Dose device. The evaluation of Quick Dose includes assessment of device operation and captures feedback on the use process, serving as a guide for enhancement of the user interface design.
After completing the usability formative evaluation, it was shown that Quick Dose can be used correctly simply by reading the instructions for use (IFU) beforehand, without complicated training (Figure 4). One of the intended user groups is laypersons, and there are no significant differences in user profiles within the layperson user group.
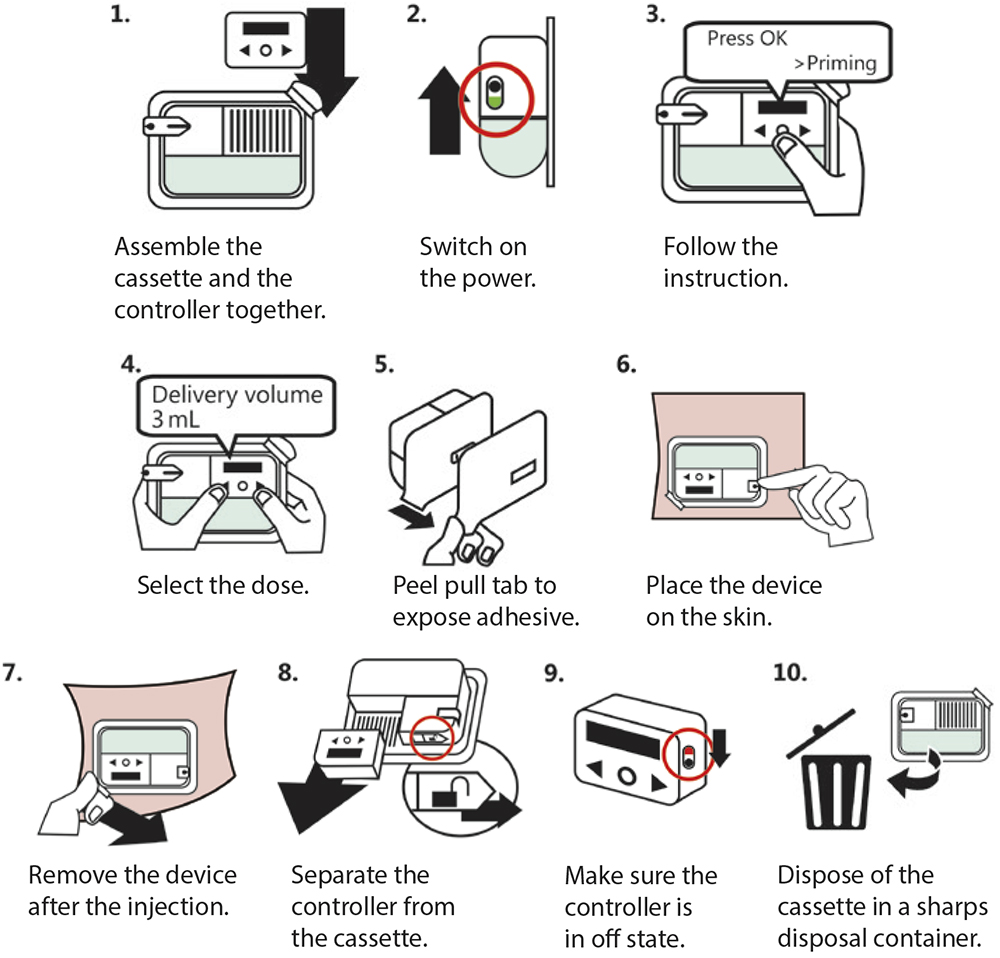
Figure 4: The Quick Dose use steps with IFU.

