To Issue 160
Citation: Giacon M, Gordon J, “Delivering on GLP-1 Demand: a Combination of Device Offerings and Supply Strategy”. ONdrugDelivery, Issue 160 (May 2024), pp 37–40.
Manuela Giacon and Josh Gordon, review the growth in demand for glucagon-like peptide-1 treatments and present Stevanato Group’s response to this exciting opportunity, showcasing its Alina® device platform for pen injectors and the Aidaptus® two-step, single-use autoinjector.
It is estimated that the glucagon-like peptide-1 (GLP-1) market will exceed US$100 billion (£80.3 billion) by 2030, driven equally by diabetes and obesity usage. In the US alone, the total number of GLP-1 users may be as high as 30 million by 2030 – that is around 9% of the overall population.1
Stevanato Group believes that, for the foreseeable future, autoinjectors and pen injectors will remain at the forefront of GLP-1 delivery. While this potential blockbuster treatment presents considerable opportunities for pharma partners, there are, of course, challenges, specifically in terms of satisfying demand.
“The pharmaceutical industry has welcomed a host of therapies and vaccines into this revered club, with indications covering everything from cancer and covid-19 to arthritis and atrial fibrillation.”
In 1986, 10 years after it was first introduced by Smith Kline & French, sales of peptic ulcer treatment Tagamet (cimetidine) surpassed the $1 billion mark, making it the first drug in history to be classified as a blockbuster.2
Since then, the pharmaceutical industry has welcomed a host of therapies and vaccines into this revered club, with indications covering everything from cancer and covid-19 to arthritis and atrial fibrillation. In recent times, however, much of the attention in the blockbuster category has been focused on GLP-1 receptor agonists (GLP-1 agonists). These drugs were originally developed for the management of blood sugar levels in people with Type 2 diabetes but have recently experienced a dramatic boom in demand as treatments for obesity.
A high-profile case in point is semaglutide. The drug was approved by the US FDA in June 2021 as a 2.4 mg once-weekly injection for chronic weight management in adults with obesity or those overweight with at least one weight-related condition, such as high blood pressure, Type 2 diabetes or high cholesterol.3 In 2023, according to market information from Midas (IQVIA), sales of semaglutide for weight management more than quadrupled year-on-year, and further interest has been spurred by new indications related to reducing cardiovascular risk, and emerging benefits are being shown in people with Alzheimer’s disease.4,5
The strength of these figures clearly demonstrates the intense demand for GLP-1 treatments, but it does not convey the unforeseen implications of such high levels of popularity. This issue came to the fore in early 2024 when the UK MHRA issued a National Patient Safety Alert triggered by global supply issues for certain GLP-1 drugs, advising healthcare professionals to conserve existing stock for patients with Type 2 diabetes.6,7 The implementation of such measures ensures that the critical priority of patient needs continues to be met, while also avoiding patients seeking what the WHO describes as “falsified or substandard” versions of these medicines through unregulated outlets on the internet.8
Looking to the future, demand for GLP-1 drugs is expected to remain high as healthcare systems look to manage obesity and diabetes among an ageing population. By 2026, anti-diabetics are expected to attract the third highest level of spending after oncology and immunology treatments. And with more than half of the global population (51% or over 4 billion people) predicted to be either overweight or living with obesity by 2035, investment in obesity drugs is forecast to rise by a compound annual growth rate of 35%–38% between 2023 and 2027.9,10
While some GLP-1 treatments currently in the pipeline are set to reach the market in oral tablet form, autoinjectors and pen injectors currently at the forefront of GLP-1 delivery will continue to deliver multiple patient benefits across this significantly expanded market segment.
For pharma companies aiming to satisfy this surging demand, the choice of delivery mechanism and delivery partner will have implications for supply chain capacity and resilience. Expediting such a device to market requires assessment and resolution of risk in areas such as drug containment and device specification as well as scaled-up production, assembly, fill-finish and distribution. Access to proven technologies, relevant device options, global manufacturing expertise and go-to-market knowledge are, therefore, all integral to supporting drug companies’ commercial strategies with a time-efficient and cost effective route to market.
Stevanato Group combines global manufacturing reach with an extensive product portfolio, allowing the company to support pharmaceutical partners with an end-to-end approach at scale. For applications suited to pen injectors, Stevanato Group’s Alina® device platform offers partners a customisable approach to the delivery of variable and multidose treatments (Figure 1). The pen injector’s performance, features and intuitive handling are compatible with established therapeutic regimens as well as innovator drug therapies that require a multidose delivery device for conditions such as diabetes and weight management. Alina® has been designed with patient convenience and confidence in mind. It features an ergonomic form and intuitive delivery mechanism, with patients receiving visual and audible feedback for clear confirmation of dose setting, correction, and injection.
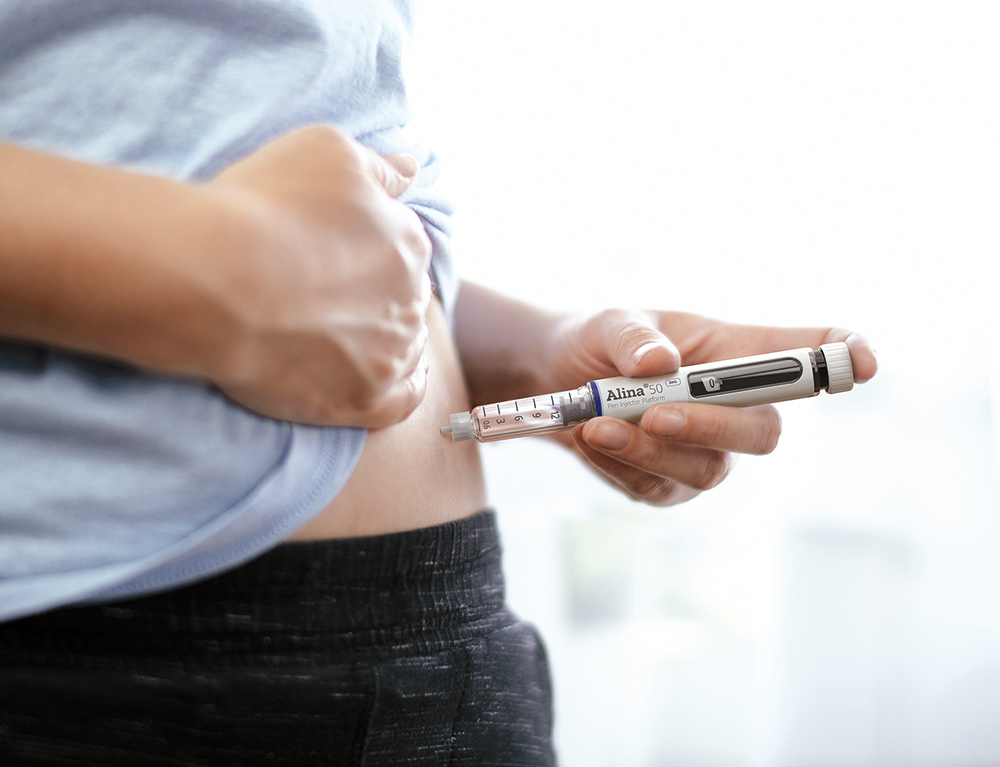
Figure 1: Stevanato Group’s Alina® device platform offers partners a customisable approach to the delivery of variable and multidose treatments.
From a manufacturing perspective, Alina® devices are built around the concept of flexibility. Production is handled at Stevanato Group’s FDA-inspected facility in Germany, providing customers with access to dedicated, established tooling and sub-assembly lines that can accommodate levels of customisation according to a customer’s production requirements. This ensures devices comply with the stringent quality standards demanded by regulatory authorities while avoiding the requirement to engage in a highly bespoke device design, prototyping and testing development process, along with the establishment of tailored tooling and assembly lines. As such, the Alina® platform provides a cost-effective and time-efficient pathway to take GLP-1 pen injector devices from bench to large-scale production, factoring in changing volume demands as a product scales throughout its development lifecycle.
In the autoinjector segment, Stevanato Group’s collaboration with Owen Mumford Pharmaceutical Services (OMPS) opens the door to next-generation single-use applications via the Aidaptus® device platform (Figure 2). Intended to balance innovation and familiarity, Aidaptus® is a two-step, single-use autoinjector with a versatile hybrid design that can accommodate both a 1 and 2.25 mL prefilled syringe in the same form factor. The platform’s novel plunger rod technology facilitates flexible filling with no change of parts, allowing for a single device to accommodate multiple dose configurations, consolidating SKUs (stock keeping units).

Figure 2: Aidaptus® is a two-step, single-use autoinjector.
Patients using Aidaptus® benefit from a familiar, intuitive delivery mechanism, while compatibility with Stevanato Group’s 29G special thin-wall needle allows for a reduction in the time and force required to inject viscous solutions. The benefits of this novel component, which are accentuated in applications involving large-molecule biologics, can be seen in an improvement in the overall patient experience when delivering GLP-1 therapies. This is achieved by expanding the internal diameter of the needle while maintaining the same external dimensions as a standard thin-walled needle, ensuring compliance with the ISO 9626:2026 requirements for the manufacture of hypodermic needles and medical devices.
With Aidaptus®, Stevanato Group’s understanding of the critical area of drug containment is brought to the fore through compatibility of the platform with Nexa® prefilled glass syringes. Supplied pre-sterilised, Nexa® high-performance syringes have been developed to ensure compatibility with drug delivery devices to meet the growing demand for drug products that patients can self-administer from the comfort of their own homes. Together, these innovative packaging solutions offer unrivalled quality and reliability for even the most sensitive and complex drug formulations.
“Stevanato Group can offer in-house expertise in the design and delivery of highly efficient and flexible assembly production lines, which can deliver time and cost savings for pharma partners in various scenarios.”
For drug owners and manufacturers, the Aidaptus® plunger rod’s ability to automatically self-adjust to the drug fill volume during final assembly, without requiring any part changes or associated process steps, streamlines the final assembly operation. Additionally, the device’s inherent flexibility supports various dose configurations earlier in the clinical development process (e.g. dose-finding studies) and commercial ambitions as part of a lifecycle management programme, both of which help to accelerate time to market.
In the case of both Aidaptus® and Alina®, individual device capabilities are married to the breadth of Stevanato Group’s service portfolio and comprehensive product knowledge in drug delivery. This unified, single-point-of-contact approach brings significant production benefits to pharma partners through minimising the number of friction points that can be present in a more fragmented supply chain and ensuring that all components ultimately knit together into a coherent combination product. This is evident with Alina®, which, while optimised for Stevanato Group’s range of Nexa® glass cartridges, is fully compatible with ISO 3 mL glass cartridges and can be integrated into a variety of fillfinish systems. Likewise, the Aidaptus® platform is compatible with all equipment suppliers with the associated format parts, allowing final assembly of the device to be integrated into existing CDMO manufacturing structures.
Where required, however, Stevanato Group can offer in-house expertise in the design and delivery of highly efficient and flexible assembly production lines, which can deliver time and cost savings for pharma partners in various scenarios, including where final assembly must be completed at a customer site or at their CDMO. Here, Stevanato Group’s in-house capabilities allow for device production equipment to be entirely designed, developed, fabricated and commissioned by the company’s own engineers, limiting the complexity and risks that can arise when engaging with multiple third parties. Additionally, the core and consistent assembly technology that spans from the company’s smaller benchtop assembly unit to its higher commercial assembly platform facilitates efficient industrialisation scale-up, which is advantageous for existing and new entrants in the GLP-1 space.
Looking to the future, Stevanato Group also offers pharma companies long-term support for drug delivery devices through its investment plan to further strengthen the company’s supply offering in multicavity tooling, injection moulding and sub-assembly offerings at its locations worldwide. Furthermore, Stevanato Group’s relationship with OMPS is an example of how partnerships and collaboration can add further layers of market-leading expertise to the company’s offering.
While such investments cement Stevanato Group’s commitment to deliver complex products at a truly global scale, its underlying objective is to reduce the number of engagement points across the combination product value chain to help pharma partners meet the sizeable and sustained demand for GLP-1 products. In a more fragile world, where geopolitical instability has the potential to cause knock-on effects for commercial activities, Stevanato Group’s highly integrated service offering and global reach can provide greater control and introduce higher levels of efficiency and continuity of supply throughout the supply chain, from sourcing raw materials through to the robust production of patient-friendly autoinjectors and pen injectors that are fully compliant with current regulatory requirements.
To find out more about the role of Stevanato Group’s device platforms in delivery GLP-1s, visit: www.stevanatogroup.com.
REFERENCES
- “The increase in appetite for obesity drugs”. J.P. Morgan, Nov 9, 2023.
- “Tagamet: The Discovery of Histamine H2-receptor Antagonists”. ACS, accessed Apr 2024.
- “FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014”. Press Release, US FDA, Jun 4, 2021.
- Hopkins Tanne J, “Wegovy: FDA approves weight loss drug to cut cardiovascular risk”. BMJ, 2024, Vol 384, article: q642.
- “Novo Nordisk”. Web Page, Alzheimer’s Disease International, accessed Apr 2024.
- National Patient Safety Alert. UK NHS, Jan 3, 2024.
- Shortage FAQs. Diabetes UK, Mar 20, 2024.
- “Shortages impacting access to glucagon-like peptide 1 receptor agonist products; increasing the potential for falsified versions”. News Publication, WHO, Jan 29, 2024.
- World Obesity Atlas 2023. Web Page, World Obesity, accessed Apr 2024.
- “The Global Use of Medicines 2023 Outlook to 2027”. Research Report, IQVIA, Jan 18, 2023.

