To Issue 168
Citation: Brandes N, Allmendinger A, Mahler H-C, “Device Selection: Injection Volumes for Subcutaneous Administration”. ONdrugDelivery, Issue 168 (Jan 2025), pp 66–70.
Nicolas Brandes, Andrea Allmendinger and Hanns-Christian Mahler explore the critical considerations for subcutaneous injection volume, beginning with the historical and scientific perspectives on injection volume before delving into the challenges of determining volume ranges and their associated fill volumes.
INTRODUCTION
The subcutaneous (SC) route is increasingly becoming the preferred route for the administration of biologics, especially in the context of patient-centric care. The advantages of SC injections are well documented – ease of self-administration, opportunities for at-home use and the avoidance of intravenous (IV) delivery – and contribute to an improved patient experience and lower costs for the healthcare system. Additionally, there may be pharmacological benefits for SC administration, such as when targeting the lymph nodes. All these benefits are supporting a growing market of drugs delivered via SC administration, not just as a therapeutic option for patients but also as a strategic choice for pharmaceutical companies during drug development.1
Determining the optimal dose volume for SC delivery is challenging, particularly in development and early clinical phases. Early in development, the “commercial patient dose” – the dose intended for the marketed product – is not yet defined in most cases. This uncertainty is due to several factors, including the evolving understanding of the molecule’s pharmacokinetic (PK) profile and efficacy, as well as the clinical programme’s goals. Decisions about final dose and injection frequency are often a combination of the needs of the scientific drug product and the commercial market strategy. For instance, therapies aiming to reduce injection frequency, such as from weekly to monthly, often require higher injection volumes or more concentrated formulations, which may introduce technical challenges. At the same time, larger doses may enhance patient comfort due to the reduced number of injections.2

Figure 1: Example container (syringe) and autoinjector device, used for delivering injection volumes up
to 2.25 mL.
The physical and chemical properties of a drug product significantly influence the feasibility for SC delivery. Factors such as viscosity and stability play an important role in determining whether a molecule can be concentrated into a volume suitable for SC injection. Formulation decisions also directly affect the choice of the delivery device. Low-viscosity formulations align with prefilled syringes, needle safety devices and standard autoinjectors (Figure 1), whereas highly viscous drug products may require an enabling technology that is capable of delivering the drug at an acceptable injection force and speed. Other considerations for dose and device selection include the clinical and regulatory context, the target indication and target users, and the competitive environment. For example, needle size heavily impacts usability (injection forces) and is specifically critical for highly viscous formulations.
In early clinical trials, dosing flexibility is key, as formulation and volume often evolve alongside emerging clinical data.3 However, as SC administration becomes more prevalent, drug development must also address practical constraints such as manufacturing scalability, patient experience and regulatory requirements, ensuring that the selected approach is both scalable and market ready. These factors underline the importance of balancing scientific, commercial and patient requirements.
SC INJECTION VOLUME
Historical Perspective on Volume Tolerability
An SC delivery volume of 1–2 mL has been traditionally considered a common target (and assumed limit) for self-administration, with higher SC volumes historically believed to be unpleasant for patients and to lead to local tissue reactions, with no controlled studies or assessments available. Healthcare professionals were cautious about SC injections exceeding 1–2 mL, stating concerns about pain, swelling and erythema at the site of injection. These limitations were further aggravated by the absence of advanced devices capable of delivering larger volumes effectively – SC injections were mainly done manually or using small-volume prefilled syringes and autoinjectors.
“Larger volumes can be injected into the SC tissue, as demonstrated in clinical studies.”
However, perceptions began to shift as systematic studies evaluated the capacity of SC tissue to accommodate larger volumes, challenging traditional assumptions. In 2015, Mathaes et al reviewed the landscape of SC injections and concluded that larger volumes can be injected into the SC tissue as demonstrated in clinical studies, with SC injections of up to 5 mL typically not being of any concern.4 These findings have broadened the opportunities for SC delivery, allowing higher-dose therapies to transition from IV infusion to SC injection.
Patient Insights
While technology has started to push the theoretical boundaries for larger-volume SC delivery, different patient studies have offered valuable insights into the practical limits of SC injection volume through assessing patient perceptions of SC injections ranging from 1 mL to 5 mL. It was concluded that higher volumes were well tolerated, especially when being delivered using appropriate injection techniques and devices. Injection speed and device ergonomics may also be relevant in mitigating discomfort, and the interplay between formulation properties and delivery technology is key.
While studies have shown the potential for larger SC volumes, physiological and practical constraints must be considered. Tissue elasticity, injection site and fluid viscosity are key factors influencing tolerability. For example, slower injection speeds and lower-viscosity formulations have been reported to reduce pain and swelling but may extend injection time, impacting patient acceptance.
Advanced Technology Supporting the Delivery of Larger Volumes
The use of permeation enhancers (PEs) such as hyaluronidase as a formulation component in SC products has been helpful in expanding SC injection limits toward 10 mL and beyond. PEs temporarily modify the extracellular matrix, degrading hyaluronic acid, thereby facilitating the dispersion of injected fluids due to diminished tissue backpressure. Nowadays, however, there is also clear scientific evidence that injection volumes of 25 mL can be injected subcutaneously without any relevant pain or injection site reactions, even in the absence of any permeation enhancers.5 Additionally, a technological advancement that has enabled the SC administration of large volumes is a novel class of large-volume autoinjectors and on-body delivery systems.
For drug product development, advanced technologies have opened up new possibilities for biologics and other high-dose therapies, allowing them to be delivered via the SC route rather than the IV route. However, introducing new device technologies comes with additional complexities, such as the need for combination product development, device complexity, regulatory considerations, higher unit cost and more complex supply chains.
“The maximum SC injection volume is not a fixed value but a dynamic parameter shaped by advancements in formulation science and device engineering.”
Looking Ahead
The maximum SC injection volume is not a fixed value but a dynamic parameter shaped by advancements in formulation science and device engineering. New technologies are continually pushing the boundaries of what is possible, paving the way for new therapeutic modalities.
It is clear that the question of how much can be injected is only one piece of the puzzle. The interplay between injection volume, formulation properties and device design underlines the complexity of SC product development and sets the stage for deeper exploration.
DECIDING ON INJECTION FREQUENCY
When considering the SC injection volume, choosing the injection frequency is another critical aspect of developing injectable drug products. In general, the less frequent the injections, the more convenient it is for patients. However, reduced injection frequency requires a higher dose per administration, which presents two significant challenges. First, higher doses necessitate either larger injection volumes or higher drug concentrations, both of which pose technical difficulties, such as minimising irritation at the injection site. Highly concentrated formulations also often lead to increased viscosity, which can complicate both the handling of the formulation and the design of the injection device.
Another key decision is whether to use single or multiple injections for treatment. During clinical studies, it must be defined whether a larger dose will be delivered in a single injection or split across multiple smaller injections. For instance, treatment could involve either a single 4 mL injection or two 2 mL injections. This decision has several implications. On the one hand, multiple injections may lead to a different bioavailability than a single injection, potentially affecting the efficacy of the treatment. On the other hand, large injection volumes can impact bioavailability, also potentially affecting the efficacy of the treatment. From a development and timeline perspective, multiple smaller injections may be necessary if high-concentration formulations are not yet available or if a suitable device-drug combination product capable of delivering larger volumes is still under development.
DECIDING ON VOLUME RANGE AND PRODUCT PRESENTATION
The selection of product presentation – prefilled syringes, autoinjectors, wearable devices or vial-and-syringe systems – must balance considerations of intended use, human factors, patient population, user preference (including options for multiple injections) and patient compliance. However, there are considerations beyond the user’s perspective comprising technical challenges related to product properties such as viscosity, device performance, compatibility with the primary packaging container, quality and regulatory aspects, cost of goods, sustainability considerations, technology maturity, supply chain security, and availability and capabilities of manufacturing partners. Furthermore, product presentations for products in clinical stages must consider additional aspects such as dosing flexibility and product losses.
Figure 2 summarises current options for selecting primary packaging containers, associated functional containers and devices depending on the intended SC injection volume (for single use). Needle selection for SC injections is typically 27G (normal or thin wall), although, in rare cases, 25G or bigger needles are used, balancing injection time against potential patient discomfort. The needle size chosen depends on the indication, patient population and competitive product landscape. The choice of needle size also determines the acceptable viscosity, and therefore the maximum concentration, of the API in its formulation (including its upper specification limit), assuming a given target of injection force for that dedicated patient or user group.
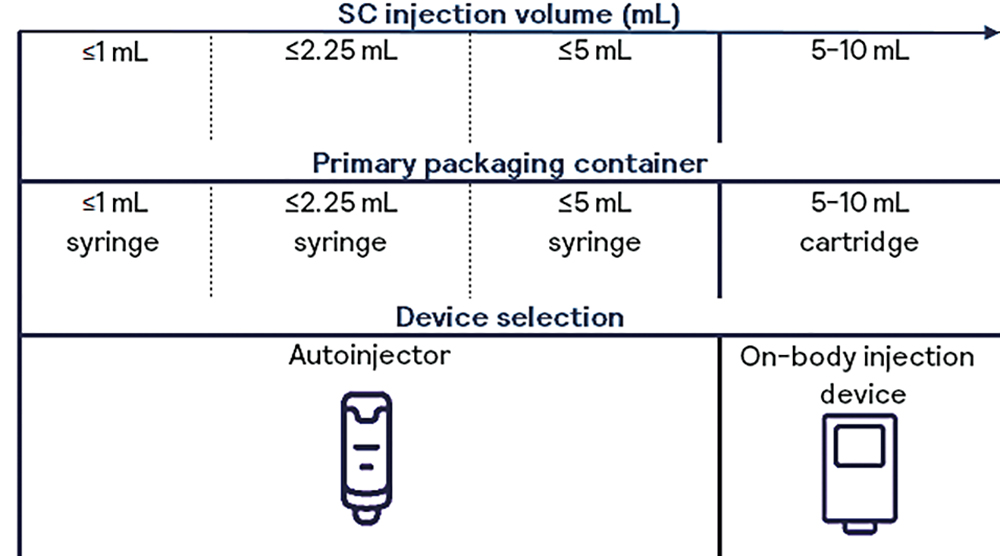
Figure 2: Selection of primary packaging containers and devices related to SC injection volume.
The concentration of a drug product is a critical factor influencing both its technical manufacturability and clinical usability and must be carefully selected. High-concentration protein formulations present significant technical challenges, including higher viscosities that complicate manufacturing processes, administration processes necessitating higher injection forces and stability challenges, such as higher aggregation propensity, accumulation of host-cell proteins that degrade polysorbates and other issues that relate to adverse product quality stability.3
Therefore, for high-viscosity products, the selection of primary packaging components is a key factor for usability. Conversely, lower-concentration formulations are easier to develop and manufacture due to improved stability and lower viscosity. However, they often present larger injection volumes or longer injection times, which may affect patient convenience. As a result, the interplay between formulation concentration and viscosity, injection volume, injection time and injection frequency introduces complex trade-offs in product development. Decisions must involve all elements, including formulation, manufacturing and device development, as well as clinical and marketing considerations. Optimising these variables is crucial to balancing clinical outcomes and the patient experience while also addressing manufacturability and stability constraints.
Selection of Fill Volume
The SC injection volume is not equal to but lower than the volume that is filled into the primary packaging material. Determining the optimal fill volume for parenteral products is a critical step influenced by multiple technical and regulatory considerations. Recent US FDA guidance emphasises minimising overfill, reducing waste during administration and drug product losses during manufacturing to improve the cost of goods and healthcare costs overall.6
Furthermore, overfilling can compromise safety by overdosing but can also lead to unacceptable and unauthorised product misuse, such as using residual formulation, possibly pooling from multiple vials, thereby increasing the risks of microbial and particulate contamination. Additionally, an excessive fill volume is economically undesirable, particularly for cost-intensive biologics.
The excess volumes are meant to be sufficient to permit withdrawal and administration of the labelled volumes accounting for hold-up volumes in the container, as well as in the syringe or needle used for administration, especially in vial presentations. It also must consider the potential variability in the extractable volume test, as well as filling technology precision. Therefore, the overfill may vary, for example, when manufacturing in multiple facilities with varying fill precision. In multi-use device presentations, additional volume must account for priming, repeated withdrawals and ensuring the correct dose for each injection, and also ensure that the dose withdrawal does not compromise sterility or stability.
“Bubble-free filling and stopper setting to ensure a product without non-essential headspace is highly beneficial.”
This is especially critical for syringes and cartridges – bubble-free filling and stopper setting to ensure a product without non-essential headspace is highly beneficial as the inclusion of air can disrupt dose accuracy, particularly in small-volume formats. This is specifically relevant for treatments that require the down-dosing of a product, in the case of multi-use applications, or for products where the product leaflet prescribes to push out the air bubble prior to injection, leading to potential error and accidental pushout of actual product. Absence of headspace can also eliminate the need for priming. Innovations in filling technologies and container design are thus essential to enable product improvements and meet evolving regulatory expectations. Consequently, strategies are needed to adequately determine and optimise fill volume operations and to meet both lower and upper fill volume limits, balancing safety, regulatory compliance and cost considerations.
WHY CHOOSE TEN23 HEALTH?
A contract design, manufacturing and testing organisation such as ten23 health is appropriately positioned to support its customers with technical and regulatory experience when taking crucial decisions for selecting product presentations, including device and primary packaging container selection for SC drug products. ten23 health’s team of experts can design an adequate strategy from early-stage development to commercialisation to de-risk the development approach.
ten23 health offers end-to-end services for sterile drug products, including IV and SC formulation development, drug device selection, integration and testing, manufacturing process development, comparability studies, analytical development, clinical and commercial GMP fill-finish and quality control release and stability testing. ten23 health provides its GMP fill-finish of complex and high-precision containers (including bubble-free filling) at its facility in Visp (Switzerland), including syringes, vials and cartridges, including the capacity to handle both glass and polymer containers on the same line. Transitioning from a vial to a syringe or cartridge configuration requires no transfers to other lines or facilities, ensuring a seamless process when integrated with the necessary technical evaluations.
REFERENCES
- Bittner B, Schmidt J, “Advancing Subcutaneous Dosing Regimens for Biotherapeutics: Clinical Strategies for Expedited Market Access”. BioDrugs, 2024, Vol 38(1), pp 23–46.
- Bittner B, Richter W, Schmidt J, “Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities”. BioDrugs, 2018, Vol 32(5), pp 425–440.
- Adler M, Reddy Goli VA, Allmendinger A, Mahler H-C, “Transitioning from Vial to Subcutaneous Injection Devices for Biological Drug Products”. ONdrugDelivery, Issue 160 (May 2024), pp 8–14.
- Mathaes R et al, “Subcutaneous Injection Volume of Biopharmaceuticals – Pushing the Boundaries”. J Pharm Sci, 2016, Vol 105(8), pp 2255–2259.
- Dang X et al, “Clinical Investigation of Large Volume Subcutaneous Delivery up to 25 mL for Lean and Non-Lean Subjects”. Pharm Res, 2024, vol 41(4), pp 751–763.
- “Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products”. US FDA Guidance for Industry, Jun 2015.

