To Issue 149
Citation: Badiei A, “Digital Twin in the Pharmaceutical Supply Chain.” ONdrugDelivery, Issue 149 (Jun 2023), pp 45–58.
Gerresheimer’s Ali Badiei explains the role of digital twin technology and its integration into the pharmaceutical supply chain, highlighting an innovative approach to ensuring drug identity and quality.
The pharmaceutical supply chain has grown increasingly complex over time, necessitating strict adherence to safety and efficacy standards. As stakeholders in the healthcare industry search for innovative solutions to address these challenges, Gerresheimer, a leading provider of healthcare solutions and drug delivery systems, is pioneering the integration of digital twin technology into the pharmaceutical supply chain. Currently, the supply chain experiences data fragmentation, with information stored in isolated silos. However, modern digital technologies and innovative partner ecosystems have the potential to securely connect all supply chain participants, creating a cohesive and trustworthy supply network.
“The benefits of digital twin technology include quality improvements throughout the supply chain and easy access
to certifications issued at various stages of the process.”
DIGITAL TWIN TECHNOLOGY IN THE PHARMACEUTICAL SUPPLY CHAIN
Gerresheimer is working to revolutionise its primary packaging by transforming it into a secure gateway to access digital twins. A digital twin is a virtual representation of a physical asset at a specific point in a process. By mirroring processes such as manufacturing steps and creating a virtual replica of an asset, like a syringe, it becomes possible to digitally trace an asset’s journey from its inception to the end of its lifecycle. This approach enables multiple stakeholders along the value chain to securely track the product’s current status and determine its true identity by digitally tracing its origin.
The benefits of digital twin technology include quality improvements throughout the supply chain and easy access to certifications issued at various stages of the process. Additionally, it helps combat counterfeiting in the highly sensitive pharmaceutical market, enabling secure and instant identification of drugs to be administered at the point of care. It also establishes a high-quality logistics chain where all items can be tracked. This enables faster root-cause analysis in the case of quality issues, as well as efficient limitation of a problem to the affected containers. A digital twin of the primary packaging will also support the prevention of mix-ups at filling operations by automatically comparing data from different production steps. This comprehensive list of benefits not only enhances the overall quality of future drug delivery but also reduces costs associated with cumbersome quality checks. It mitigates negative consequences, such as major recalls of expensive drugs or drug delivery systems.
This article will explore a concrete example of how digital twin technology can be applied from manufacturing prefilled syringes (PFSs) to administering high-quality medications. A traceability solution that demonstrates the benefits mentioned above will also be presented (Figure 1).
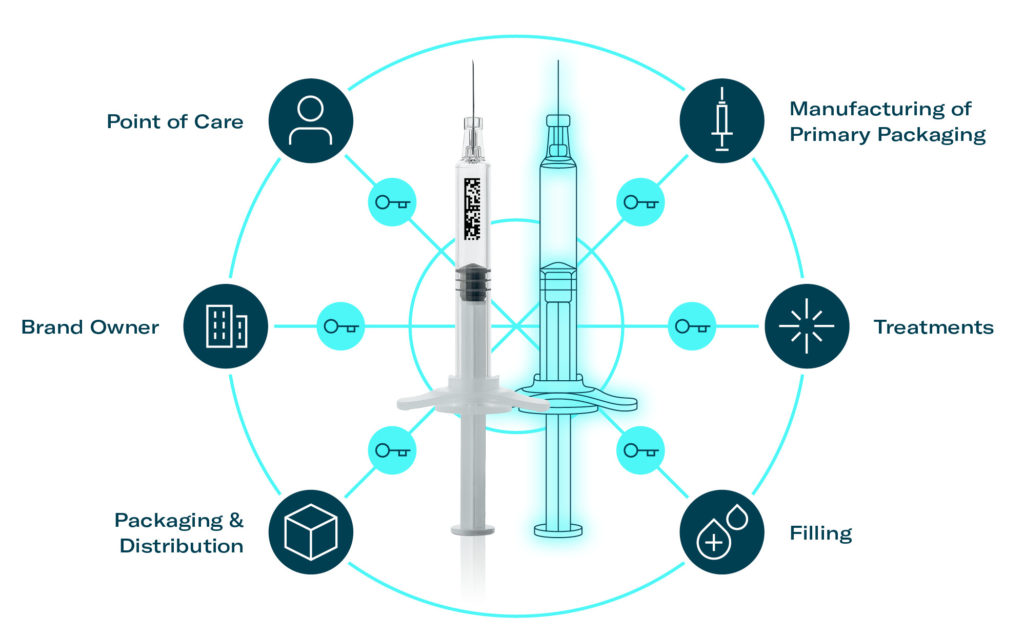
Figure 1: Digital twin in the centre of the pharmaceutical supply chain.
“The decentralised nature of a blockchain allows members to have an exact copy of the chain.”
UNDERLYING TECHNOLOGIES FOR DIGITAL TWIN IMPLEMENTATION
To create a future-proof platform on which every company in the value chain can contribute data, Gerresheimer has partnered with Merck to develop a framework based on blockchain technology. This approach enables a zero-trust level of security for creating and adding virtual assets, eliminating the need for numerous intermediaries to control data flow. The resulting data, such as the identity of the medication or the specifications of the delivery system, serve as a “single source of truth” along the value chain.
Initially, selecting a physical asset in the supply chain and determining how it will be identified are crucial steps in creating digital twins. As illustrated in Figure 2, primary packaging, such as a prefilled syringe, is an ideal candidate for virtualisation as it is the only container that “travels” along the whole supply chain from production to the point of care. Unique identification is achieved through secure and tamper-proof marking of the asset, using methods like printed data matrices or embedded radio frequency identification (RFID) tags. The data matrix contains a unique identifier (UID), similar to serial numbers, which represents an individual physical asset or batch without revealing any details. Ensuring the authenticity of an asset’s UID is also necessary, and a so-called crypto anchor is used to securely link the UID to a physical asset, preventing cloning or forgery and unauthorised transfer.

Figure 2: Unique identification on primary packaging.
When an asset receives its UID and crypto anchor during the production process (e.g. through printing straight after forming the glass body), all relevant identity data, such as physical specifications or batch information, can be digitally connected with its virtual replica on the blockchain-based decentralised data platform. The digital twin of the asset is then officially created in the chain, thereby establishing a highly secure identity. As the physical asset, such as a syringe, moves to the next player in the chain (e.g. a pharmaceutical company filling the syringe), a new information block with relevant data can be added. The addition of blocks with enriched data by new stakeholders throughout the value chain contributes to overall benefits for all parties while increasing their accountability for managing their data.
A blockchain-based information management system offers several advantages. In comparison with other technologies, blockchains offer high security, interoperability and easy scaling across many parties. In fact, the blocks (containing data) are immutable; it is only possible to create and add new blocks (data) to the chain. Each block’s authenticity is secured through the so-called hash of the previous block, ensuring that the data chain cannot be tampered with. Traceability of information back to the creation of a block is possible, and data access depends on the authorisation rights of the stakeholder accessing the data, guaranteeing data integrity.
Additionally, the decentralised nature of a blockchain allows members to have an exact copy of the chain. Transactions within a blockchain are typically handled through tokens, which function as the chain’s currency. Smart contracts facilitate self-executing transactions based on predefined rules and can be used to automate business transactions.
Due to its peer-to-peer and distributed network topology, blockchain technology offers a secure, efficient and fast means of data transaction in a decentralised manner without intermediaries that control the data flow of others. Permissioned or private blockchains, which are more common in supply chain applications, enable simpler consensus mechanisms, allowing fewer known participants to add new data blocks compared with public blockchains. Private chains are often employed to increase throughput, reduce cost and increase privacy.
“At this stage, the physical syringe can be linked to its virtual replica, or digital twin, containing relevant manufacturing information, such as specifications and quality data, like the certificate of analysis.”
THE DIGITAL JOURNEY OF A PREFILLED SYRINGE
Gerresheimer, a leading manufacturer of prefillable syringes, conducted a field trial to explore the implementation of digital twin technology in the pharmaceutical supply chain. By using advanced data-marking techniques, a physical syringe was labelled with a UID using a data matrix code compliant with ISO/IEC 16022 standards. This label underwent rigorous testing to ensure it met pharmaceutical manufacturing requirements, including durability in extreme temperatures, resistance to chemical agents and tamper-proof properties. Additionally, an embedded security code (crypto anchor) was incorporated to enable identity verification.
At this stage, the physical syringe can be linked to its virtual replica, or digital twin, containing relevant manufacturing information, such as specifications and quality data, like the certificate of analysis. This initial information forms the first data block in the blockchain system, with Gerresheimer acting as the first manufacturer in the chain. Suppliers may contribute data or certificates in the future, and the information about the physical syringe, identifiable through its UID, can be securely accessed by new stakeholders in the chain.
In a preliminary use case, Merck developed an easy-to-use mobile application on the M2M Trust Framework. The application is based on decentralised blockchain technology and can read the syringe’s UID and verify it by scanning the invisible crypto anchor. The app enables pharmaceutical firms that fill the syringe with medication to instantly confirm that all manufacturing and quality requirements have been met, significantly enhancing the efficiency of quality control and audits (Figure 3). Furthermore, pharmaceutical firms can promptly file complaints if irregularities are detected on the syringe, as the app provides the exact manufacturing specifications and batch information. This streamlined process reduces the effort required to identify the root cause of a complaint, enables faster feedback, helps avoid stockpiling batches with suspected quality issues and even prevents recalls.
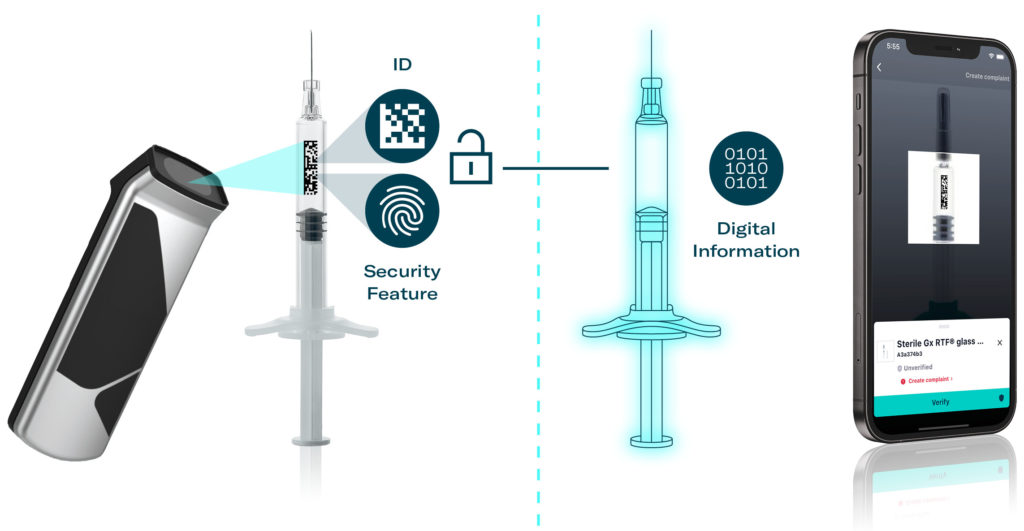
Figure 3: Information on demand through the digital twin.
Ongoing development of additional use cases and integration of advanced technologies, such as RFID for the UID in various drug delivery systems, continues to expand the digital twin ecosystem. As more partners and players join, the following benefits can be realised along the value chain:
- Improving overall quality, reducing complaint handling processes and minimising recalls
- Avoiding batch mix-ups of filled containers and enhancing batch segregation during and after the fill-finish process
- Increasing sustainability by enabling recycling and waste reduction, resulting in a positive environmental impact
- Contributing to a patient-centric pharmaceutical supply chain by allowing point-of-care facilities to instantly and securely identify the medication and support global anticounterfeiting efforts.
FUTURE-PROOF PARTNER ECOSYSTEMS AND INNOVATIONS ON THE HORIZON
Partnerships are vital for success in creating digital ecosystems. Merck and Gerresheimer are collaborating to develop a digital twin solution that enhances traceability and trust in critical steps along the pharmaceutical supply chain. Gerresheimer is committed to contributing to a patient-centric and reliable pharmaceutical supply chain by driving digitalisation using advanced technology and trusted partnerships.
As a company known for offering one of the broadest product portfolios in the market, Gerresheimer also develops various technologies for identifying its products. The company believes that different products and use cases will require distinct marking technologies. Gerresheimer has a proven digital printing infrastructure ready for sample production and is working at full speed to implement RFID for selected products, such as prefillable syringes.

