To Issue 172
Citation: Thomsen M H, “Driving Sustainability in Drug Delivery Device Design and Manufacturing”. ONdrugDelivery, Issue 172 (May 2025), pp 40–44.
Martin Høier Thomsen discusses the need for the pharma industry to rise to the challenges of meeting its sustainability goals, explaining how MGS Mfg Group’s expertise and established practices to drug delivery device design and manufacture present a forward-thinking approach to achieving this aim.
MEETING THE PHARMA INDUSTRY’S SUSTAINABILITY CHALLENGE
Sustainability is now a critical objective for the pharma industry, one that extends beyond drug formulation and into the design, production and lifecycle management of drug delivery devices. As regulatory agencies tighten environmental standards and consumers increasingly favour eco-conscious products, pharma manufacturers must find ways to align their sustainability goals with patient safety, therapeutic efficacy and global compliance.
In fact, the healthcare sector, which includes the pharma industry, accounted for 4.4% of global carbon emissions in 2014,1 a figure that has only grown alongside rising demand for advanced treatments and the widespread adoption of complex drug delivery systems. These systems, ranging from autoinjectors to inhalers, prefilled syringes (PFS) and intravenous (IV) delivery components, all play a critical role in modern medicine but also significantly contribute to environmental impact due to their material composition, manufacturing energy needs and end-of-life disposal challenges.
At the intersection of these environmental and regulatory pressures, pharma companies are seeking new strategies to reduce the carbon footprint of their devices without compromising patient outcomes or regulatory compliance. This is where MGS comes in. By applying a robust eco-design methodology (Figure 1) and employing vertically integrated manufacturing capabilities, MGS helps its pharma partners minimise waste, improve efficiency and future-proof drug delivery devices.
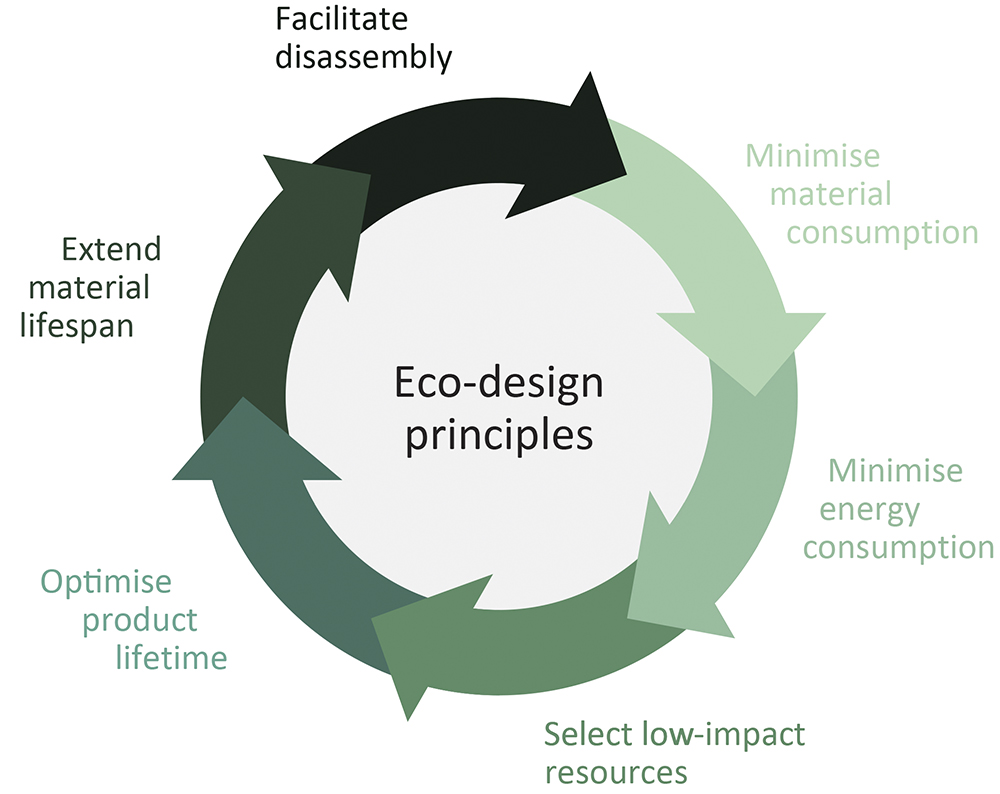
Figure 1: The six eco-design principles implemented in MGS’ product development model.
WHY STRATEGIC COLLABORATION IS ESSENTIAL
Pharma companies developing drug delivery devices are in a unique position. They operate in one of the most heavily regulated sectors globally, upholding the highest standards of quality, while simultaneously managing consumer and healthcare provider demands for environmental responsibility.
Working with a specialised manufacturing partner for drug delivery devices offers key advantages in navigating this complex landscape. By collaborating with experts in sustainable device design and production,1 pharma firms can gain access to:
- Leading Expertise and Dedicated Support: Partnering with MGS means working with a team of industry-leading engineers, designers and regulatory experts who are fully committed to customer and patient success. The company’s specialists bring decades of experience in drug delivery device development, ensuring that every project benefits from cutting-edge innovation and a patient-centric approach.
- Specialised Product Development and Engineering Resources: Expertise in balancing eco-design principles with strict pharma regulatory requirements, such as ISO 13485, US FDA 21 CFR and the EU MDR.
- Faster Time to Market: Streamlined design for manufacturing processes that reduces the need for costly reworks and accelerates commercialisation timelines.
- Greater Supply Chain Control: Vertical integration across manufacturing processes – product design and development, tooling, automation, manufacturing – enables comprehensive oversight of quality and sustainability metrics from concept through production and post-market support.
- Reduced Risk: A partner like MGS can help to mitigate common challenges related to product design, material selection, manufacturing efficiency and regulatory audits.
With a proven track record in drug delivery device design, engineering and manufacturing, MGS empowers its partners to meet rising global sustainability expectations while developing safe, high-performing and compliant combination products.
SIX ECO-DESIGN PRINCIPLES FOR SUSTAINABLE MANUFACTURING
MGS follows a structured, six-principle eco-design framework that optimises drug delivery devices for sustainability without sacrificing functionality or patient safety.
“MATERIAL SELECTION IS ONE OF THE MOST IMPACTFUL LEVERS AVAILABLE IN SUSTAINABLE DEVICE DESIGN.”
Designing for Material Reduction
Material selection is one of the most impactful levers available in sustainable device design. Using fewer materials and minimising their volume has cascading benefits throughout the supply chain, from reducing raw material demand and simplifying moulding processes to enhancing recyclability at a product’s end-of-life.
MGS encourages its customers to strategically balance critical performance attributes, such as durability, biocompatibility and sterilisation compatibility, with sustainability indicators such as carbon footprint, energy consumption and recyclability. For instance, polypropylene (PP) is a single-type polymer that offers excellent recyclability compared with more complex copolymers, while still meeting many pharma-grade requirements.

Figure 2: Evaluating a topology-optimised prototype – designing with material efficiency in mind supports lighter, more sustainable drug delivery devices.
Additionally, MGS employs advanced engineering techniques such as:
- Topology Optimisation: Using computer-aided design to distribute material only where it is structurally necessary, creating lightweight, resource-efficient components (Figure 2).
- Moulding and Filling Simulations: These simulations optimise wall thickness, gating locations and cooling pathways, significantly reducing material waste and supporting leaner production cycles.
Through thoughtful material reduction and optimisation, pharma partners can easily develop components and products that are lighter, more sustainable and more cost-effective to produce.
Lowering Energy Consumption at Every Stage
Energy efficiency is critical to a drug delivery device’s overall environmental footprint. From manufacturing to end use, reducing energy demand leads to measurable carbon reductions.
MGS integrates energy-saving strategies throughout its vertically integrated manufacturing operations, including:
- Servo-Electric Injection Moulding: This technology consumes significantly less energy than traditional hydraulic systems.
- Advanced Cooling Systems: Conformal cooling channels, thermally balanced moulds and insulated tooling reduce cycle times and limit energy consumption.
- Optimised Assembly Lines: Applying design for manufacturing and assembly principles, MGS engineers simpler device designs that reduce assembly complexity and energy input during production.
Additionally, MGS works with its pharma partners to design drug delivery devices that are compatible with energy-efficient sterilisation and maintenance processes, such as low-temperature sterilisation methods, further reducing a device’s lifecycle impact.
Prioritising Sustainable and Low-Impact Materials
Material selection extends beyond volume reduction to include sourcing and processing. MGS helps its pharma clients identify and incorporate low-impact materials, such as bio-based polymers and recyclable resins, into device designs where appropriate. While regulatory constraints currently limit the direct use of recycled resins and many bio-based polymers in drug delivery devices, there are still ways to integrate sustainability, such as selecting materials with lower environmental footprints or improving recyclability through mono-material design.
Beyond just recommending eco-friendly materials, MGS leverages lifecycle screenings (LCS) to assess a product’s environmental footprint early in the development process (Figure 3). LCS provides data-driven insights into the environmental impacts of material choices, enabling companies to make informed decisions that consider both regulatory requirements and sustainability goals.
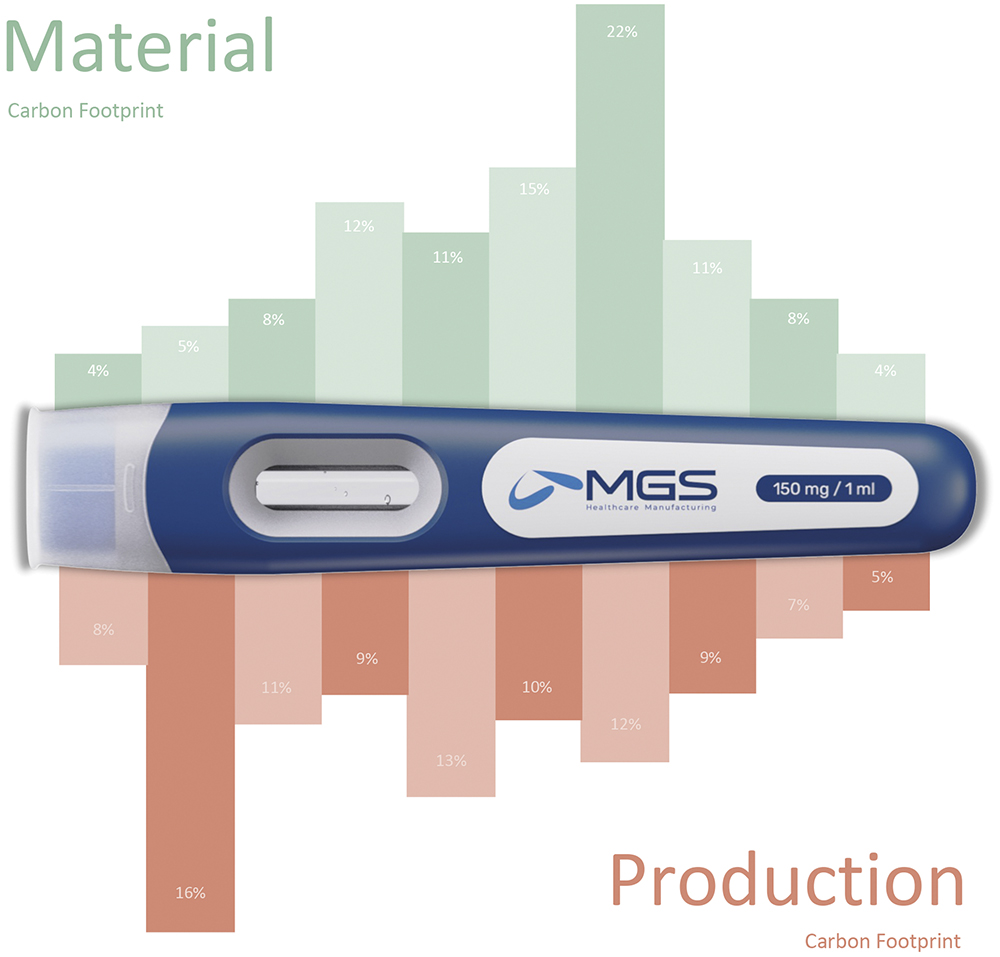
Figure 3: LCS are used to calculate a product’s carbon footprint to support design decisions.
For instance, selecting a simpler, mono-material polymer over a blend not only reduces energy during material processing but also facilitates future recycling and reduces regulatory complexity associated with waste handling and disposal.
Designing for Longevity
The pharma industry faces unique challenges when choosing between single-use and reusable devices. Single-use devices, such as PFS, IV bags and infusion tubing, must be lightweight and minimise waste at the end of their lifecycle. In contrast, reusable components, such as surgical devices or certain autoinjector housings, should be engineered for long-term performance, repair ability and durability.
MGS helps its pharma customers carefully define device lifespans and tailor designs to meet their specific use-case scenarios while avoiding overengineering. The company employs strategies that include:
- Robust Design Principles: Simplifying mechanical features and reducing component count without sacrificing reliability.
- Modular Components: Creating devices with replaceable or serviceable parts to extend product lifespan where appropriate.
- Material Durability: Selecting high performance materials that withstand repeated use, sterilisation cycles and transport handling.
By aligning design intent with actual product use requirements, pharma companies can reduce the environmental impact of both single-use and reusable devices.
Promoting Proper Recycling of Pharmaceutical Devices and Materials
Unlike consumer goods, pharmaceutical devices often cannot incorporate recycled materials due to the strict health and safety regulations that govern them. However, proper disposal and recycling of these devices remain crucial in reducing their environmental impact (Figure 4). A few ways to extend material lifespan include:
- Designing devices that use single type polymers, such as PP, to simplify recyclability
- Co-operating with material processing companies to establish take-back programmes to ensure responsible end-of-life processing of devices
- Advising on sustainable packaging solutions such as bio-based or recyclable packaging that reduce overall waste.
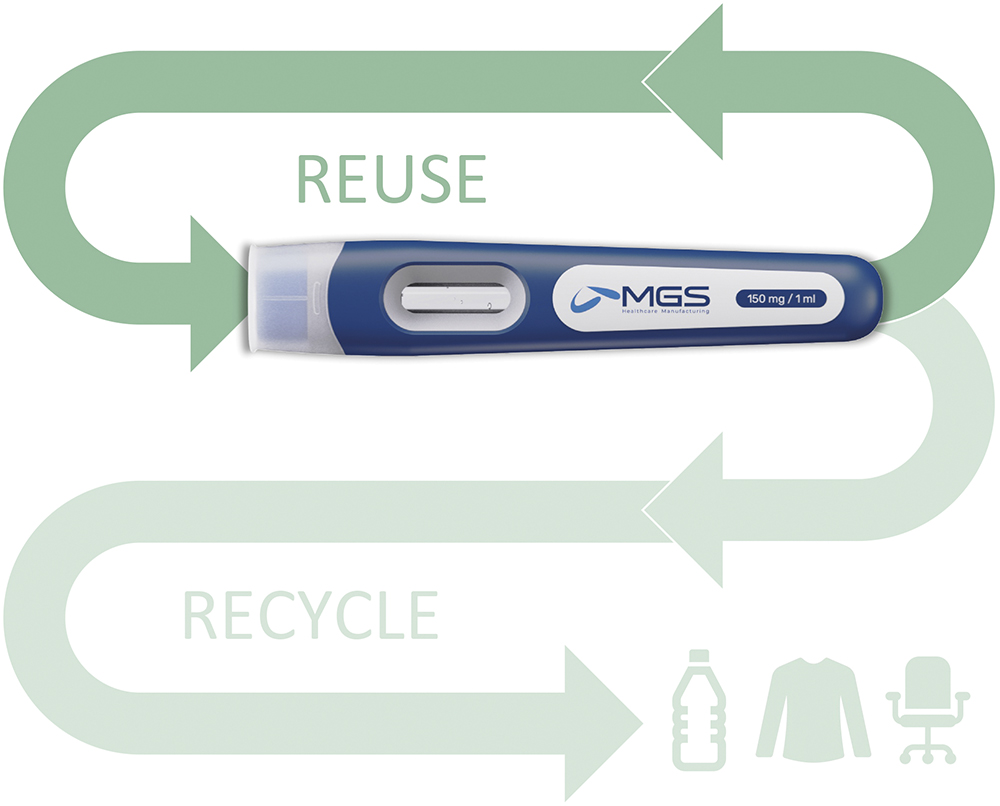
Figure 4: Important design decisions to extend material and product lifetime are made in the early development phases. The goal is to prioritise reuse and, when that’s not feasible, to ensure recyclability wherever possible.
“EASE OF DISASSEMBLY IS A KEY FACTOR IN FACILITATING THE RECYCLING OF DRUG DELIVERY DEVICES, PARTICULARLY THOSE USED IN CLINICAL OR AT-HOME CARE SETTINGS WHERE HEALTHCARE PROVIDERS AND PATIENTS HANDLE DISPOSAL.”
While closed-loop recycling for pharma-grade materials is still emerging, these strategies position leading pharma innovators to meet regulatory and corporate sustainability objectives today, while also preparing for future advancements in material recovery.
Enabling Easy Disassembly for Improved Recycling
Ease of disassembly is a key factor in facilitating the recycling of drug delivery devices, particularly those used in clinical or at-home care settings where healthcare providers and patients handle disposal. MGS integrates features into device designs that enable easier material separation post-use (Figure 5), including:
- Snap-fit connections and interlocking components that allow for manual or mechanical disassembly
- Break lines or fracture points that enable controlled breakage, which aids in separating materials for recycling
- Crush-separation designs for single-use devices that will be processed in industrial recycling facilities.

Figure 5: Hands-on evaluation of a device prototype with modular components designing for easy disassembly supports more efficient recycling and sustainable end-of-life processing.
By engineering for disassembly, MGS makes it easier for healthcare facilities, recycling centres and even patients to engage in sustainable disposal practices, helping pharma companies meet their environmental commitments.
WHY SUSTAINABLE DESIGN PROVIDES A COMPETITIVE EDGE
Eco-design isn’t just about environmental stewardship; it also offers significant business benefits:
- Operational Cost Savings: Leaner material use, shorter cycle times and reduced energy consumption help to lower production and operation costs.
- Regulatory Resilience: Early adoption of eco-design principles can help to future-proof products against tightening global environmental regulations.
- Enhanced Market Trust: Sustainability is increasingly a key purchasing factor for healthcare providers and patients alike.
By integrating sustainability into their device design and manufacturing strategies, pharma companies can position themselves as forward-thinking, responsible organisations in a competitive and evolving marketplace.
THE FUTURE OF DRUG DELIVERY DEVICES
As industry expectations shift towards greater environmental accountability, sustainability will become a baseline standard, not a differentiator. Pharma companies that proactively embrace eco-design principles will be better positioned to meet evolving regulatory frameworks and changing market demands.
MGS is committed to staying at the forefront of this transformation. The company is well positioned to help its pharma partners create next-generation drug delivery devices that deliver both clinical performance and environmental responsibility. Sustainable drug delivery device design is a necessity for modern pharma companies that are committed to environmental stewardship and regulatory excellence. By prioritising eco-design, pharma can deliver safer, greener products that help patients, providers and the planet.
MGS: A STRATEGIC PARTNER FOR SUSTAINABLE INNOVATION
MGS works with leading pharma companies to embed sustainability into every stage of device development and manufacturing. The company’s approach includes:
- End-to-end integration of eco-design principles, ensuring that sustainability is built in from concept through commercialisation
- Vertically integrated capabilities, giving its pharma customers direct access to product design and development, tooling, automation and manufacturing to drive efficiency and reduce supply chain complexity
- A continuous improvement mindset, with ongoing investments in green technologies, sustainable materials research and process optimisation.
REFERENCE
- Pichler PP et al, “International comparison of health care carbon footprints”. Environ Res Lett, 2019, Vol 14, art 064004.

