To Issue 137
Citation: Ben-David O, “Drug Delivery Devices Throughout the Drug Development Cycle”. ONdrugDelivery, Issue 137 (Sep 2022), pp 28–32.
Ori Ben-David discusses the considerations that must be made over the course of injectable drug development and how Eitan Medical’s Sapphire™ and Sorrel™ platforms offer pharmaceutical companies a full suite of device tools from early discovery through to commercialisation.
THE DRUG DEVELOPMENT PROCESS
As colleagues in the pharmaceutical industry can attest to, there are multiple stages in the drug development cycle, ranging in length and complexity, all of which must be passed successfully prior to commercialisation and market introduction of a new drug product (Figure 1). In the early discovery and development stages, a pharmaceutical company will conduct the necessary research to develop a new chemical or biological entity, initially testing it in preclinical animal studies, followed by three phases of clinical trials to determine the safety, efficacy and therapeutic effects of the medication. Following the successful completion of clinical studies and subsequent clearance by the relevant regulatory authorities, the drug can then be prepared for commercial launch.
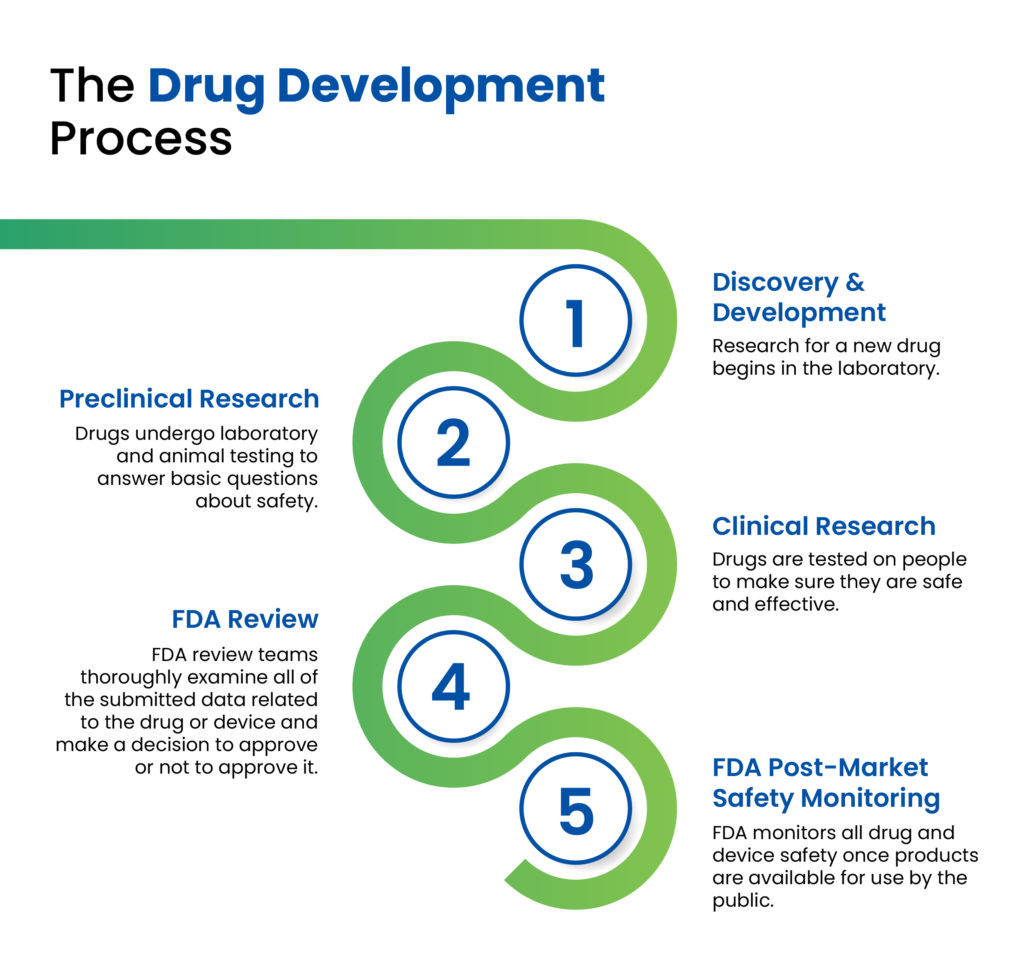
Figure 1: The drug development process as outlined by the US FDA.1

Figure 2: A key factor in the development of an injectable therapy is consideration of which primary container and associated drug delivery device will be most appropriate for the drug product. Image courtesy of Ompi, Stevanato Group.
“For injectable medications, an additional consideration must be addressed throughout the development and clinical phases – how should the drug product be administered?”
However, for injectable medications, an additional consideration must be addressed throughout the development and clinical phases – how should the drug product be administered? At each stage of the drug development cycle for an injectable medication, pharma companies must consider which primary container and drug delivery device is most appropriate to store and administer the medication (Figure 2). This is necessary to ensure a smooth drug development process through clinical trials and all the way up to commercialisation, not only introducing to market a therapeutically beneficial medication, but also an overall positive and convenient drug delivery experience for the patient.
THE EVOLUTION OF PRIMARY CONTAINERS THROUGHOUT DRUG DEVELOPMENT
During the early discovery and clinical trial phases, pharmaceutical companies generally use glass vials as the primary container for their drug product. This vial will not necessarily be the primary container for the final commercial representation but is chosen due to the wide availability of ready-to-use, off-the-shelf vials and the availability of supporting filling lines. Due to their popularity, vials are manufactured in large quantities and therefore have a very low cost per unit compared with cartridges or prefilled syringes (PFSs).
However, using a vial for commercial representation does not come without its challenges. Using a simple syringe to extract drug product from a vial generally requires multiple accessories, including syringes, needles and vial adapters, and is generally only performed by trained healthcare professionals, caregivers or experienced patients. Additionally, in general, because vials are non-collapsible drug reservoirs, the orientation of the vial must be maintained as the drug is drawn out into a drug delivery device.
As an alternative to vials, primary containment manufacturers have recently started bringing more options for cartridges and PFSs to market, which are designed to offer a more patient-centric, easy-to-use solution for drug delivery. However, the move from vials to cartridges and PFSs is a significant investment for pharmaceutical companies, considering all the necessary validation activities required for transitioning from one primary container to another. In many cases, this will also require collaboration with an additional manufacturer and possibly a dedicated development project, which will further add to development costs and increase time to market.
Regardless of the ultimate choice for a primary container, pharmaceutical developers will need to test, challenge and validate the drug product’s stability and compatibility with the primary container for the full shelf life of the drug.
THE INTEGRATION OF DEVICES THROUGHOUT THE DRUG DEVELOPMENT PROCESS
In parallel to considering the question of primary container, a pharmaceutical company must address the topic of the drug delivery device. There are multiple considerations for choosing a drug delivery device. These differ and evolve as the drug product moves through the early discovery phases, then preclinical and clinical phases, and ultimately prepares for commercial launch. During the initial stages, the availability and configurability of a drug delivery device is key. Researchers need access to solutions for administering drugs and examining the effects of different injection volumes, flow rates and viscosities on the subcutaneous tissue. As drug parameters are not yet determined during these early phases, companies generally prefer to avoid investments in customised devices at this stage.
Accordingly, the early development stages generally benefit from professional, off-the-shelf, software-controlled infusion pumps with a rich feature set. Such devices with a detailed event log and options for connectivity can assist early stages and clinical studies by allowing for automatic recording and documentation of configured parameters and events throughout the infusion. The additional benefits of connected drug delivery devices in terms of recording and monitoring are especially profound during decentralised and hybrid clinical trials, taking the burden and responsibility of self-reporting out of the study subjects’ hands, instead moving it to an automated, controlled and reliable method.
As the drug development process continues through the clinical research stage and prepares for Phase III trials and commercial launch, the pharmaceutical manufacturer must shift its focus towards considering the ideal commercial representation of the drug product once it is on the market. Injectable medications are generally launched as combination products, with an inseparable device constituent launched with them to enable drug administration. Accordingly, choosing a commercial drug delivery device and a device partner is not an easy task. Often, dedicated device teams within pharmaceutical companies are tasked with this challenge, acting as liaisons between the drug product and pharmaceutical manufacturer on the one hand, and a drug delivery device manufacturer on the other.
One consideration for choosing a commercial drug delivery device is the intended patient and user population for the device. While in the development and clinical stages, the user of the drug delivery device is generally a researcher or healthcare professional; however, if the device is intended for self-administration, it would require an assessment of the target patient population. Would the average patient be a child or an adult? Does this patient population suffer from vision or dexterity issues relating to their disease, and would those issues affect their ability to operate a device? Are these patients with chronic diseases that can be trained and familiarise themselves with the use of a more complex device over time, or are these patients that require a simple and easy-to-use device as a priority? Do these patients generally have familiarity with drug delivery devices and medical technology, or should it be assumed that this self-administration device would be a new concept for them?
A second leading consideration, tied to the first, is the intended use environment in which the drug product is to be administered. Is this drug product intended to be administered in a homecare environment? Is a healthcare provider or caregiver expected to be present? This, too, will dictate the required user interface, use steps and maximum level of complexity of the drug delivery device. As the move towards home-based care continues to develop following the covid-19 pandemic, devices that can administer medication outside of traditional clinical settings and without requiring additional supervision are seen as the future of drug delivery.
In parallel, a landscape assessment should be conducted to analyse the drug delivery devices associated with competing or similar drug products on the market. In certain cases, for generic or biosimilar drug products, the device experience would need to mimic that of the originator drug, so that patients transitioning from one drug product to its generic or biosimilar version would essentially have the same drug delivery experience. For competing molecules, pharma companies may look for a competitive edge when introducing a drug delivery device to the market, such as a wearable device, a smartphone app that is able to track the injections or a sleek and discrete new device design.

Figure 3: Eitan Medical’s flagship Sapphire™ infusion pump.
OPTIMISING THE ROAD TO COMMERCIALISATION WITH A DEVICE PARTNER
As the road from discovery to commercialisation of an injectable medication is intertwined with drug delivery devices along the way, selecting a reliable, flexible and versatile device partner is key. Eitan Medical’s flagship product is the commercialised Sapphire™ infusion pump (Figure 3). This pump is small in size yet big in performance, offering tailored solutions for a variety of infusion needs, including hospitalisation, ambulatory and home infusion, all in a single pump.
The Sapphire™ family of infusion solutions is based on patented magnetic flow control and advanced technologies aimed at enhancing patient safety and reducing total cost of ownership. These devices use a colour touchscreen, allowing for a quick setup and intuitive workflows, and support a wide range of flow rates, positioning it as the ideal, flexible off-the-shelf solution for clinical research phases.

Figure 4: Eitan Medical’s Sorrel wearable drug delivery platform.
Eitan Medical’s Sorrel™ wearable drug delivery platform is ideally positioned for large volume injections in the homecare environment (Figure 4). The Sorrel™ device is a subcutaneous wearable injector that is simple to use and easy to scale up with configurations for both prefilled and preloaded cartridges and vials, as well as a device filled at the point of care by a syringe (Figure 5). With the Sorrel™ device, pharmaceutical developers can use the primary container of their choice. The unique electromechanical primary agnostic pumping mechanism provides the flexibility to allow Sorrel™ devices to accommodate a wide range of vials and cartridges, minimising the risks, cost and time associated with the development of a new delivery system or primary container.
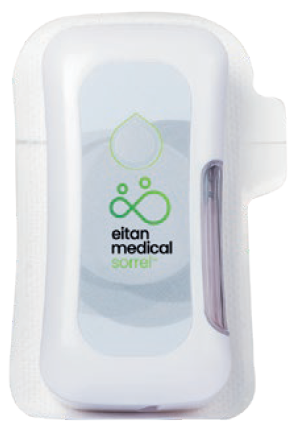
Figure 5: The Sorrel™ can be configured for use with prefilled and preloaded cartridges and vials, as well as filling at point of care with a prefilled syringe.
Taken together, this means that Eitan Medical’s pharmaceutical partners can use a range of products throughout the drug development process.”
Taken together, this means that Eitan Medical’s pharmaceutical partners can use a range of products throughout the drug development process – the Sapphire™ infusion pump in early discovery stages, a Sorrel™ vial-based wearable injector for clinical phases, and either a Sorrel™ vial-based or cartridge-based wearable injector for commercial use. Doing so can provide both the company and the end user with greater flexibility, control and ease of use. Additionally, innovative new technologies are in development allowing for lyophilised medications to be used within a Sorrel™ wearable injector, potentially introducing the use of convenient-to-use wearable injectors to even more drug products and markets.
Furthermore, the recently introduced Eitan Insights™ digital health platform will provide clinicians and homecare providers with remote treatment data and visibility of Eitan Medical’s suite of advanced infusion and drug delivery devices. The system will track patient treatment status, as well as the device’s location and status, allowing clinicians to assess events and conduct remote follow-ups on treatment progress. Aggregated patient data and cloud-based processing will allow caregivers to identify treatment patterns and provide data-based adjustments. Eitan Medical is envisaging a future with connected health, flexibility and usability at the forefront, putting patients, physicians and pharma at the heart of drug delivery.
REFERENCE
- “The Drug Development Process”. US FDA website, accessed Sep 2021.

