To Issue 128
Citation: Fuchs C, Buchner S, “eFlow Technology Nebulisers with Digital Therapy Management: the PARI Connect Eco-System”. ONdrugDelivery, Issue 128 (Dec 2021), pp 10–14.
Carola Fuchs and Simon Buchner describe PARI’s new nebuliser and digital Connect eco-system and how it supports patients, directly and indirectly, through carefully considered device usability in combination with digital therapy management solutions.
“PARI has recently updated the eBase and eTrack Controllers, with a special focus on improving usability and implementing better therapy support features.”
While it is obvious that successful inhalation therapies require efficacious drugs, they are also heavily reliant on efficient delivery systems that address a patient’s ability to follow their treatment regime. The easier it is for a patient to follow their treatment regime and use their device, the more likely it is that their therapy will be effective. The usability of a device can affect the delivered dosage in a given therapy session, as well as overall therapy acceptance in the long run. Despite being an important factor for positive real-world outcomes, therapy acceptance and adherence have been found to be as low as 36% for some inhaled therapies.1
According to internal market research with over 250 patients, physicians and pharma company employees, both device usability and the possibility to transfer and share data between a patient and their physician are key features for improving the success of inhalation therapies. As such, connectivity for providing feedback on patients’ inhalations will have a significant role to play in the sector going forward.
THE PARI CONNECT ECO-SYSTEM
PARI is a provider of customised vibrating membrane nebulisers, supporting its clients during the clinical development, regulatory approval and commercial phase of their product development. PARI’s devices always consist of a drug-specific, customised nebuliser handset and a controller. Depending on the target patient population and their specific needs, three types of controller are available:
- eLete®, without any display or connectivity functionality
- eBase®, with a display but without connectivity functionality
- eTrack®, a Bluetooth and wi-fi ready controller.
PARI’s nebuliser devices are complemented by digital supporting products that are part of the PARI Connect eco-system – a proprietary app that enables data transfer from eTrack Controllers to secure cloud storage coupled with the PARItrack Dashboard, which aggregates and displays the data stored in the cloud (Figure 1).
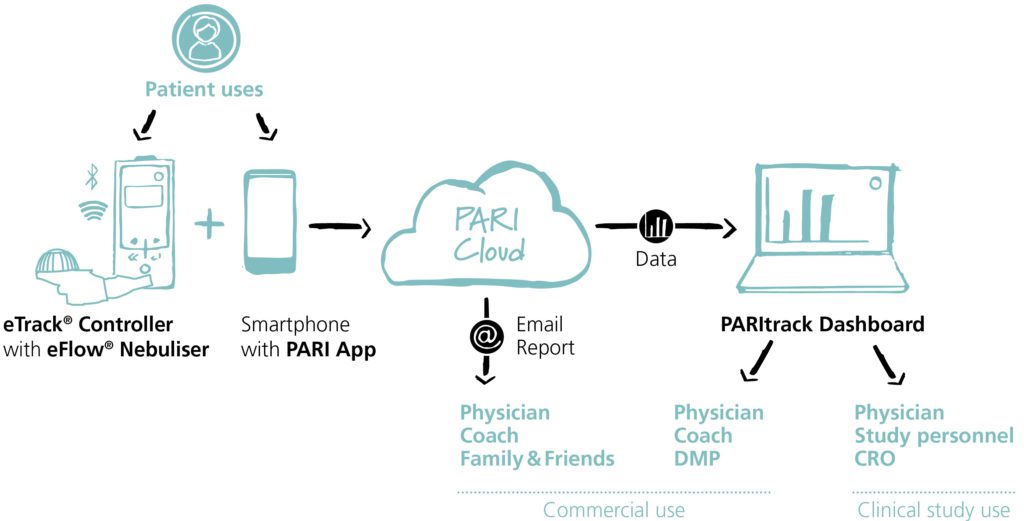
Figure 1: eTrack Controller in the PARI Connect eco-system.
“With the progress in digitisation in other areas of their lives, the expectations patients place on digital tools in the medical field is rising.”
PARI’S NEW CONTROLLERS FOR eFLOW® TECHNOLOGY NEBULISERS
PARI has recently updated the eBase and eTrack Controllers, with a special focus on improving usability and implementing better therapy support features. The new eTrack Controller, together with the corresponding app, will be available in summer 2022 (Figure 2). This update focused on human-centric design by following a comprehensive human factors engineering process – not only to comply with regulatory requirements but also to ensure safe and effective use, and a positive user experience that enables a smooth transition into future partner collaborations and clinical studies.

Figure 2: The new eTrack Controller with eFlow Technology nebuliser and PARI Connect app.
Knowing that there is a broad range of potential patient populations for future drug and device programmes, PARI tested the device setup across a range of indications throughout the development phase, including considerations of respective limitations that may be concomitant with certain respiratory diseases. The challenge was designing a device that enables patients with a wide range of user characteristics, such as age, physical limitations or limited experience, to effectively perform their therapy and, simultaneously, create a user interface that implements connectivity using cutting-edge digital tools.
General improvements were made that emphasise the mobility and user-friendliness of the device, for example, both controllers had their weight and size significantly reduced, alongside the addition of an integrated rechargeable battery pack that is designed to last for 90 minutes after three years of normal use. Further to this, the data transfer feature of the new eTrack Controller was improved by implementing both Bluetooth and wireless LAN connectivity. This feature allows for the automatic transfer of nebulisation data without the need for the patient to actively transfer the data after each treatment, enabling regular, instant data sharing with a telemedicine centre or a physician. Additionally, an inhalation detection function was implemented in the eTrack Controller to optimise the monitoring feature.
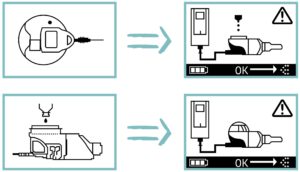
Figure 3: Evolution of exemplary error screens – current controller (left) versus new controller (right).
PARI also focused on the re-design of the user interface. The target was to develop self-explanatory pictograms that are readily comprehensible without additional text. To this end, simplified lateral graphics of device components were used to inform the user about device status or malfunction (Figure 3).
Another challenge was to design buttons that are robust and easy to clean on the one hand, but that allows users with limited dexterity, or even a tremor, to successfully push them on the other. The use of a keypad that covers the front of the controller makes cleaning and disinfection methods, such as chemical wipe disinfection, as simple as possible. Again, this requires finding the optimal haptic feedback, and so the push forces required by the controller buttons were iteratively adjusted according to user feedback. During the early development stage, participants that are familiar with the current eFlow Controller gave input regarding their expectations on a new device. As a result, PARI incorporated a settings menu within the new interface that allows patients to adapt their therapy feedback to their needs. For example, some users described being annoyed when constantly receiving audio feedback and would prefer to mute the device, whereas parents were positive about the way that audio feedback kept them informed while their child was performing a therapy. In the later stage of human factors engineering, users were observed in comprehensive simulated-use scenarios, including performing a complete therapy to validate the safe and effective use of the device. PARI also tested if users were able to connect to the eTrack Controller via an app. By iterative adaption of the user interface, PARI developed a simple setup process that connects the nebuliser to the app using only the symbols on the eTrack Controller. Once the connection is established, further configurations can be done within the app itself. This allows PARI to offer different app functionalities and levels of complexity. On the one hand, simple automatic data transfer to the PARI cloud ensures a successful transfer for clinical studies and patients with limited affinity to digital tools. On the other hand, detailed analysis and visualisation of nebulisation data can be offered to patients wanting to get more involved.
During the development of the PARI Connect system, the accompanying app also underwent human factors evaluations. This started with remote user tests on basic wireframes to create a clearly arranged user interface in the app, after which further studies were performed to observe users entering and managing therapy plans and extracting reports, as well as analysing the effect on adherence. By doing so, PARI was able to focus on the essential user needs for daily therapy management – throughout this process PARI always kept the benefits for the patient in mind.
“The PARItrack Dashboard is a web portal for physicians to access the data transferred from the app and the connected devices, based on six years’ experience with use of eTrack Controller and PARItrack within clinical trials with more than 2,000 patients across Europe,
the US, Canada and the UK, available for both commercial and clinical settings.”
PARI CONNECT® APP
With the progress in digitisation in other areas of their lives, the expectations patients place on digital tools in the medical field is rising. Chronically ill patients appreciate a digital tool that helps to collect all relevant information about their disease. Thus, the new eTrack Controller can be connected to an app on a patient’s mobile phone to document all nebulisations and information related to their disease.
The PARI Connect app has the following features:
- A comprehensive plan encompassing all therapies, such as pills, inhalation and physiotherapy
- Individual reminders for all therapies
- Automatic transfer of data from the eTrack Controller and a home spirometer to the PARI cloud
- Manual documentation of vital parameters, such as lung function, glucose, oxygen saturation and BMI
- Both short daily queries and validated quality-of-life questionnaires
- Graphical visualisation of stored therapy data to see long-term trends for adherence and vital parameters
- Exportable reports for physicians, coaches, parents and friends
- Automatic message generation in case of decreased adherence or lung function to a selected person
- Diary function
- Support from family and friends (“Buddy-System”).
When opening the app, the patient immediately sees the next therapy they need to take and can track the nebulisations that they have already done, as was shown in Figure 1. For use in clinical trials a special version, PARI Study Connect, is available to consider corresponding requirements.
Data is collected, encrypted, securely transferred and stored in the PARI cloud considering all requirements for data privacy according to the EU General Data Protection Regulation. For commercial use, the patient has control over who they share their data with. They can either share a report via email with any person of their trust or give access to their data to their physician via the PARItrack Dashboard. Within a clinical study, the data are shared according to the study protocol, which needs to be incorporated into the patient information and consent form. The app is a tool for documenting and transferring data and therefore not a medical device from a regulatory perspective, however, this might change in the future if more features are integrated.
PARITRACK DASHBOARD
The PARItrack Dashboard is a web portal for physicians to access the data transferred from the app and the connected devices, based on six years’ experience with use of eTrack Controller and PARItrack within clinical trials with more than 2,000 patients across Europe, the US, Canada and the UK, available for both commercial and clinical settings. PARI understands that to maximise the benefit of using the system, it is important that physicians have easy access to aggregated data and can gain a good overview with only a quick check.
When accessing the PARItrack Dashboard, physicians can immediately see all their patients alongside their therapy adherence data and, if applicable, can also see their vital parameters, allowing them to focus on the patients that most need support (Figure 4). Additionally, they can investigate the details of each of their patients, featuring lists with all the aggregated information on inhalation sessions, including the date, time and duration of each nebulisation, the switch-off criterion, the corresponding drug and the graphical analysis of therapy adherence. Notifications for drops in adherence or lung function can be adjusted and activated.
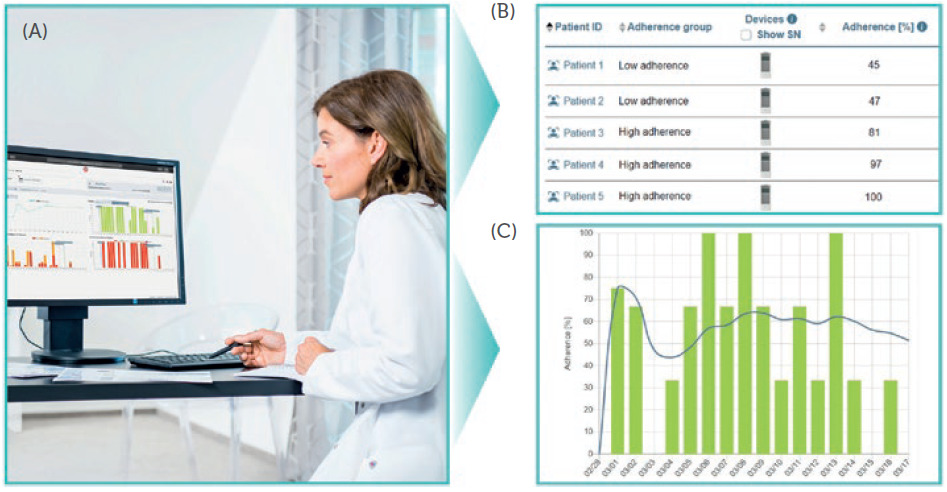
Figure 4: PARItrack Dashboard gives access to the data for the physician and helps to focus on the patients that need support: web portal (A), list of patients (B), exemplary adherence report (C).
In a commercial setting, an attending physician can set up their practice in the PARItrack Dashboard and invite their patients with an eTrack Controller to share their data. If a patient agrees and decides to share their data, the data will be available in the PARItrack Dashboard, but the patient can withdraw whenever they so wish. Data access can also be given to a physiotherapist, a therapy coach or nurses as part of a telemedicine programme. For use in clinical studies, the structure of the PARItrack Dashboard reflects the organisation of patients in study centres, with different access roles for physicians, study personnel, contract research organisations and sponsors.
The advantage of this system is that the setup and technical solution for use in clinical studies is the same as for commercial use, thereby enabling an easy transfer from use within clinical development to the commercial phase. Storing the data in the secure PARI cloud allows country-specific requirements to be met easily with local branches in the corresponding countries. If necessary, this also enables worldwide use of the system.
BENEFITS OF ADHERENCE MONITORING AND THERAPY MANAGEMENT SUPPORT
The benefits of using the system in clinical trials have been demonstrated in recent years. Patients who are less adherent can be directly contacted by clinicians and other healthcare professionals to assist with device handling or for training and motivation. This has resulted in very high mean adherence rates – between 99 and 76% for studies with a duration of two to six weeks and up to two years, respectively.2 Monitoring also supports improved evaluation of study outcomes with respect to the efficacy and safety of a medication by correlating efficacy with adherence. In total, this can result in cost and time savings as higher rates of non-adherence necessitate greater numbers of participants and elongated recruitment periods.3
eTrack Controllers have also been used within studies considering the adherence of chronically ill patients in respect to their daily treatments in real-world settings. The adherence to standard therapies is known to be approximately 34%. Recent research shows that it is possible to achieve significantly higher objectively measured adherence to inhaled medications (sustained over 12 months) with an absolute difference of 18% at 50 weeks in the largest self-management intervention trial in cystic fibrosis (CF) to date (607 adult participants, 19 UK-based test centres).4 Interestingly, the intervention group showed a lower perceived CF treatment burden than the control group with usual care and lower adherence. This is the only trial thus far to demonstrate a sustained difference in adherence versus a control group using a theory-based approach including habit formation. eTrack Controllers with eFlow Technology nebulisers were used in this trial to accurately record participants’ inhalations. Intervention participants additionally had access to the CFHealthHub digital platform via an app where they saw their adherence data alongside motivational and educational material.
OUTLOOK
In another ongoing multi-centre study sponsored by the innovation grant of the German statutory health insurances, telemonitoring adherence in combination with lung function, together with coaching of non-adherent patients by staff trained in patient psychology, is being evaluated with respect to adherence increase, health outcomes, quality of life and economic impact in a randomised controlled trial.5 The study is using PARI’s eTrack Controller, the PARI Connect app and the PARItrack Dashboard.
REFERENCES
- Daniels T et al, “Accurate Assessment of Adherence: Self-Report and Clinician Report vs Electronic Monitoring Of Nebulizers”. Chest, 2011, Vol 140(2), pp 425–432.
- Koehler Y, Fuchs C, “Monitoring Nebuliser Usage & Lung Function in Clinical Trials”. ONdrugDelivery Magazine, Issue 87 (Jun 2018), pp 66–70.
- Alsumidaie M, “Non-Adherence: A Direct Influence on Clinical Trial Duration and Cost”. Applied Clinical Trials, Apr 2017.
- Wildman MJ et al, “Self-management Intervention to Reduce Pulmonary Exacerbations by Supporting Treatment Adherence in Adults with Cystic Fibrosis: A Randomised Controlled Trial”. Thorax, 2021, Online as thoraxjnl-2021-217594.
- Thee S, “A Multi-Centre, Randomized, Controlled Trial on Coaching and Telemonitoring in Patients with Cystic Fibrosis: conneCT CF”. BMC Pulm Med, 2021, Vol 21, Article 131.

