To Issue 164
Citation: Chu L, “Enabling Large-Volume Subcutaneous Injection with a Smart Wearable Injection Platform”. ONdrugDelivery, Issue 164 (Sep 2024), pp 60–64.
Leonard Chu presents Altek Biotechnology‘s Alby injection platform and highlights its versatility as a delivery platform for large-volume subcutaneous injections.
Over the past few years, interest in large-volume delivery by self-administration has increased, with a growing number of large-volume subcutaneous (SC) injectables having been commercialised – whether the drug was originally formulated as a large-volume SC injectable or was converted from intravenous injection to SC injection.
“By considering the needs of various stakeholders, a smart wearable injection platform may provide a solution to large-volume on-body delivery.”
CONSIDERATIONS FOR LARGE-VOLUME ON-BODY DELIVERY
Wearable injectors play a key role in the self-administration of large-volume drugs (defined here as 3 mL or greater), which leads to the question of what to look for in a wearable injector that would increase value to the stakeholders. For patients, device safety, usability and price are important. For pharmaceutical companies, speed to market, risk management, drug compatibility, safety, clinical outcomes and the availability of fill-finish lines are some of the top considerations. And for device companies, quality, cost, regulatory compliance and manufacturability are some of the key considerations, to name a few. By considering the needs of various stakeholders, a smart wearable injection platform may provide a solution to large-volume on-body delivery.
THE ALBY INJECTION PLATFORM – DOES “ONE SIZE FIT ALL”?
Altek’s Alby (top image) was designed as an injection platform intended to cover a wide range of large-volume on-body delivery needs. However, does the concept of “one size fits all” apply to Alby? Fundamentally, Alby shares the same concept and many identical parts across the different models in the platform. As a result, Alby can easily adapt to a different set of delivery requirements by only making the essential changes, with minimal impact to the overall architecture. Let us take “delivery volume” as an example. A standard 10R vial-based injector that covers a delivery volume range of 5–10 mL can easily be reconfigured into a 20R injector covering 11–20 mL by simply changing the size of the device housing while keeping the majority of components unchanged. In other words, pharmaceutical partners can harness the versatility of Alby to achieve a more efficient development timeline and a lower development cost across different drug candidates in the pipeline.
Any delivery requirements beyond the pre-assumed specification range can be discussed on a case-by-case basis, including customisation options. Despite the commonality offered by the platform concept, Alby injectors have plenty of room for branding and differentiation.
FLEXIBLE CHOICE OF PRIMARY CONTAINER WITHOUT DRUG TRANSFER
Cartridges are commonly seen in self-injection devices such as autoinjectors or pen injectors. On the other hand, while glass vials are the most commonly used containers for packaging pharmaceuticals, they are rarely integrated into self-injection devices. For the self-administration of large-volume drugs, Alby is designed to accommodate both container types.
“The design of Alby avoids the need to perform drug transfer from the primary container to a secondary reservoir inside or outside the device, especially when dealing with vials.”
Prior to injection, users need to load the container into the injector. There are several benefits to this “plug and play” concept. First, users can access and clean the stopper or septum of the container for disinfecting the container stopper-piercing needle interface. Second, no refrigeration is required for the transport and storage of the devices, thus lowering the cost of the logistics value chain. The design of Alby also avoids the need to perform drug transfer from the primary container to a secondary reservoir inside or outside the device, which is especially valuable when dealing with vials. Eliminating drug transfer further reduces the risk of user handling errors and the cost associated with drug transfer systems. Furthermore, Alby does not have a secondary reservoir that would require additional drug compatibility studies.
THE BENEFITS OF DIGITAL INJECTORS
Electronics technology enables numerous functions and capabilities that are critical to injection device safety and performance. Taking advantage of the electronics technology, Alby is a versatile delivery system that can perform SC injection in a controlled manner, provide detailed information about dosing status and enable key safety features that prevent device misuse. One example of this versatility is the electromechanical drive system, which can generate a specific dosing profile based on a combination of pre-programmed delivery rates and dosing intervals. The data generated from the use of the injectors can also be stored and shared anonymously with users and healthcare professionals (HCPs) for enhanced safety and disease management.

Figure 1: The user interface of Alby injectors.
The user interface of Alby is designed for simplicity, as shown in Figure 1. Upon placing the injector on the body, there is only one visible button to start the delivery. There are three LED lights that provide the visual signals, each with a distinct purpose of informing users about the delivery status, the battery status or the Bluetooth connectivity status. In addition to the visual feedback, all Alby injectors have an internal speaker that provides an auditory output.
“The reusability of electronic parts could therefore have the potential to drive the “cost per injection” significantly lower.”
Electronics have already become affordable to the general public thanks to the advancement of technology. Electronics-enabled features, such as the drive system, safety reminders and other dummy-proof functions, could replace or reduce some of the single-use plastic components that would otherwise fulfil those functions. The reusability of electronic parts could therefore have the potential to drive the “cost per injection” significantly lower.
DEVICE SELECTION BASED ON DOSE FREQUENCY
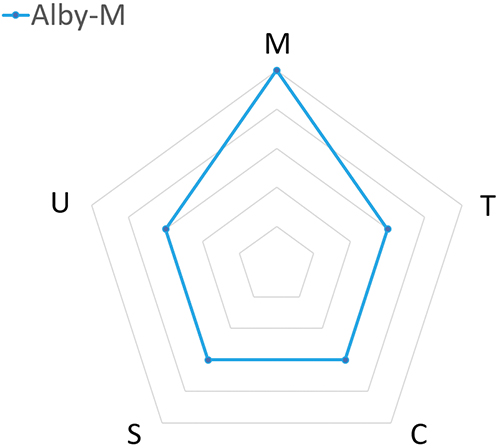
Although the versatility of Alby offers a wide selection of injector models, which model best meets the dosing needs? To assist the decision makers, a radar chart is provided for each injector model that is designed to address a specific range of dose frequency. Table 1 compares the attributes (manufacturability, time to market, cost savings per injection, sustainability and ease of use) between three Alby injector models: Alby-M-R, Alby-M and Alby-S. This analysis only serves as a guideline for choosing a suitable injector model, rather than an instruction for selection.
| Model | Alby-M-R | Alby-M | Alby-S |
| Vial and Cartridge Injectors |  |
 |
 |
| Suggested Dosing Frequency | High Frequency | Medium Frequency | Low Frequency |
| (M) Manufacturability | High | High | High |
| (T) Time to Market | Slower | Intermediate | Faster |
| (C) Cost Savings per Injection | More | Intermediate | Less |
| (S) Sustainability | Higher | Intermediate | Lower |
| (U) Ease of Use | More user steps | Some user steps | Fewer user steps |
| Comparison of Attributes Between Three Models | 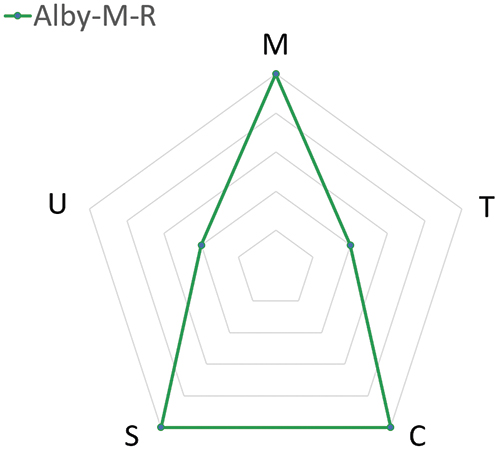 |
 |
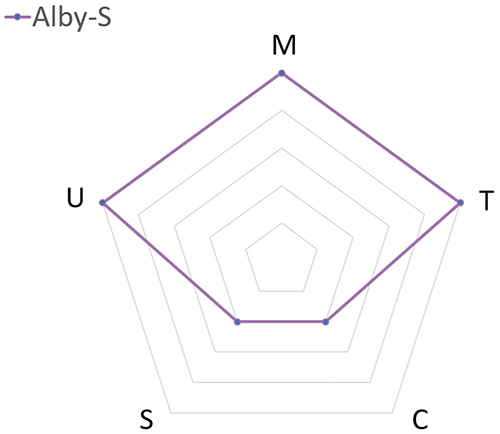 |
Table 1: An analysis comparing Alby injector models designed for different dose frequency needs.
- Alby-M-R: A multi-use rechargeable device that consists of a reusable unit and a disposable unit designed for high-dosing frequency (i.e. ≥1 dose/week), providing the best value for the lowest cost per injection and the least amount of medical waste
- Alby-M: A multi-use device that consists of a reusable unit and a disposable unit designed for a moderate-dosing frequency (i.e. once every two weeks to three months), allowing a balanced benefit from great usability to a lower cost per injection
- Alby-S: A single-use, fully disposable device designed for long-acting drugs with low-dosing frequency (i.e. once every three months or longer), giving excellent usability and the fastest time to market.
DOSE ACCURACY TEST
When it comes to evaluating the feasibility of an injection device, dose accuracy is identified as a key performance function according to ISO 11608-1:2022, EMA guidelines and US FDA Guidance for Industry.1–3 The purpose of performing in-use tests under different atmospheric conditions is to ensure that the dose accuracy is kept within the target of ±5% for large-volume delivery, as users may experience some variation of temperature while wearing the devices.
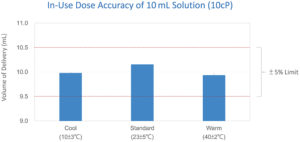
Figure 2: In-use dose accuracy test at different temperatures (N=5).
Alby’s vial-based wearable injectors were selected as the test model and were subjected to cool, standard and warm atmospheric conditions (N=5). The dose accuracy tests were based on the delivery of 10 mL of a 10 cp sucrose solution into the air from a 10R standard vial.4 The delivery rate was set to approximately 2 mL/min. The injectors, along with the vials, were preconditioned in the test chamber under cool (10˚C ± 3˚C) or warm (40˚C ± 2˚C) conditions, with the humidity set at approximately 50%. For the standard atmosphere condition (23˚C ± 5˚C), the tests were conducted in an office environment with air-conditioning. Delivered sucrose samples were collected and weighed. As shown in Figure 2, the dose accuracy of 10 mL delivery at various temperatures was kept well within 5%.
The injection time was also reasonably precise within ±30 seconds for a 10 mL delivery of a viscous solution. Here the term “reasonably precise” in the context of injection time is a subjective judgement when comparing the time variation of Alby injections with the assumed time variation of manual injections by HCPs (actual data are not available).
In addition to in-use testing in cool, standard and warm atmospheres, dose accuracy tests were performed using different solutions of different viscosities. Other functional tests included needle insertion and retraction, alarm systems with visual and audio feedback, and various device safety features. These laboratory tests generated supporting evidence for the feasibility of the Alby injection platform, and more tests are being conducted to increase confidence in the device design, usability and manufacturability.
SMART INJECTORS WITH CONNECTIVITY
Wireless connectivity is gaining relevance in the field of drug delivery. The covid-19 pandemic has accelerated the growing acceptance of self-administration. However, self-administration without connectivity is like using a computer without an Internet connection – while the primary tasks of delivering a drug can be performed, valuable information regarding device performance and patient compliance cannot be effectively stored and shared for disease management.
“Alby injectors are smart devices featuring connectivity such as Bluetooth or near-field communication.”
To enable telemedicine coupled with self-administration, Alby injectors are smart devices featuring connectivity such as Bluetooth or near-field communication. As shown in Figure 3, Alby’s vial-based injectors have successfully demonstrated wireless functionality by transferring the history log of the dosing process to an Android demonstration app via Bluetooth. The device’s function and performance metrics were captured and transferred to the app, including the sequence of operation, exact timing and the corresponding device operation, such as device activation, start of delivery, progress of delivery, end of delivery or an error report. Other device operations not shown in Figure 4 can be written in the history log according to the design requirements.
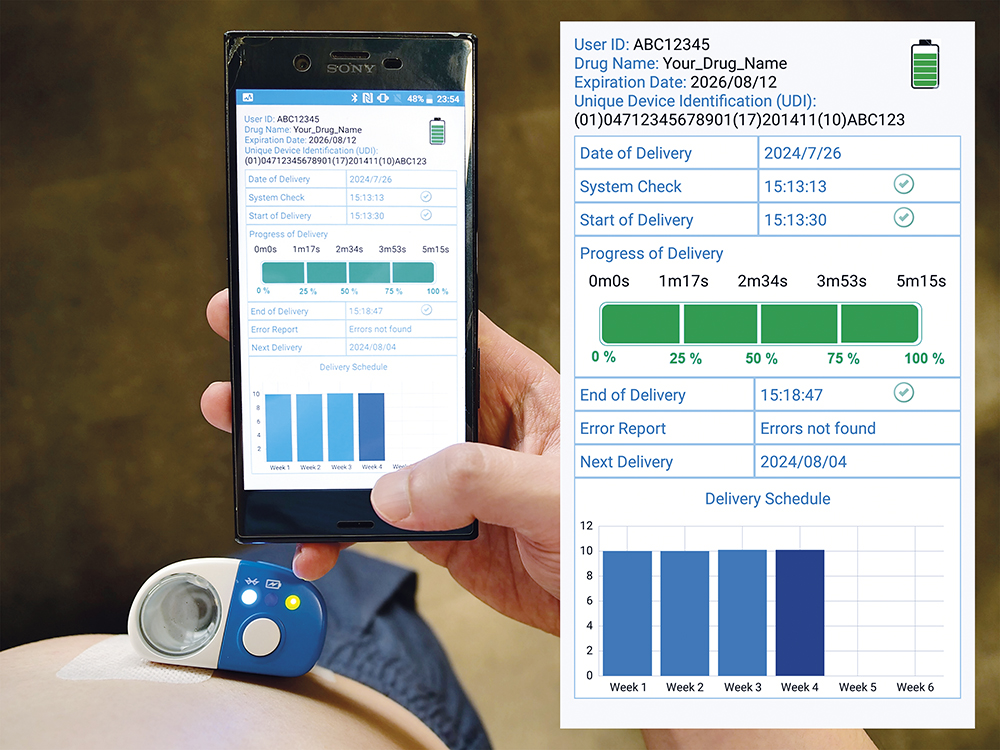
Figure 3: Demonstration of digital health management by Alby.
To protect patient privacy, the data can be recorded in an anonymous manner where only authorised HCPs can match the data to the patient. Besides logging the dosing data for an enhanced self-administration and disease management experience, the app could also send reminders to patients before it is time to receive the injection. With the aid of connectivity, Alby can offer a more informative and interactive experience to users and HCPs.
THE ENABLER AND PRODUCT DIFFERENTIATOR
Alby is a versatile injection platform that can deliver large volumes of drugs, connect wirelessly via Bluetooth and accommodate different types of primary containers without the need for drug transfer. In other words, Alby could serve as a starting point for one drug candidate and extend to others with minimal customisation, which reduces both time and cost of development and commercialisation. The platform’s accurate dosing at different temperatures provides further support for the feasibility of Alby.
Alby, as both a large-volume delivery enabler and a pharmaceutical product differentiator, is now ready to be combined with real drug candidates to validate its performance in preclinical studies, and subsequently clinical trials and commercial use.
REFERENCES
- “ISO 11608-1:2022. Needle-based injection systems for medical use – Requirements and test methods – Part 1: Needle-based injection systems, fourth edition”. International Standards, 2022.
- “Guideline on the quality requirements for drug-device combinations (draft), 2019”. European Medicines Agency (EMA).
- “Guidance for Industry (draft guidance): Essential Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products”. US FDA, 2024.
- Swindells JF et al, “Viscosities of Sucrose Solutions at Various Temperatures: Tables of Recalculated Values”. Supplement to National Bureau of Standards Circular 440, 1958, pp 2–7.

