To Issue 166
Citation: Jansen-Otten C, “FluroTec® Prefillable Syringe Plungers: A Solution for Low-Temperature Storage”. ONdrugDelivery, Issue 166 (Oct 2024), pp 102–105.
Christa Jansen-Otten discusses how West Pharmaceutical Services‘ FluroTec® plungers for prefilled syringe solutions can cope with cold storage conditions to extend drug shelf life and preserve integrity.
Selecting the appropriate primary container for drug delivery is an important decision that can directly impact patients. Prefilled syringes (PFSs) are one of the fastest growing segments within the injectables market, driven by a strong biologics pipeline, combination products, home care and self-administration.
There are many reasons why pharmaceutical companies are keen to switch from multidose vials (MDVs) to a safe PFS. One is to create a more convenient patient-centric administration format – PFSs are filled with an accurate dose and therefore reduce the risk of medication errors in a ready-to-use format. Using PFSs reduces the risk of microbiological contamination due to them requiring fewer preparation steps and less time for healthcare providers to prepare the drug for injection. In addition, PFSs also have less overfill compared with MDVs, which reduces drug waste.
During the covid-19 pandemic, various sizes of MDV formats were approved by regulatory authorities, which have proven to be a highly effective containment solution, enabling high vaccination rates (13.5 billion vaccine doses administered worldwide through August 2023, according to the WHO in August 2021); however, a transition towards single-dose applications, such as PFS container solutions, during the endemic phase might be appropriate.
The development of messenger ribonucleic acid (mRNA) vaccines has paved the way for a whole new class of promising vaccines for infectious diseases and cancer, mRNA treatment for food and environmental allergies, and a platform for genetic diseases.
One of the challenges with highly sensitive mRNA-based vaccines is that they must be maintained at low temperatures. While data exists for cold storage in MDVs, less data is available for PFSs, which serve as both a container for storage and the drug delivery device. Therefore, PFSs must be carefully evaluated to high standards regarding key performance metrics, such as the container closure integrity (CCI), break loose and extrusion forces, and plunger movement during cold storage.
“Given the need for more high-value drug products that can be stored at low temperatures to extend shelf life, preserve drug integrity and provide patient convenience, PFSs appear to be a logical solution.”
Given the need for more high-value drug products that can be stored at low temperatures to extend shelf life, preserve drug integrity and provide patient convenience, PFSs appear to be a logical solution – for room temperature storage, they are perfect. However, drugs that need sub-ambient storage come with additional concerns, including leakage, syringe cracking and sterility breaches by plunger movement. These concerns are independent of the level of siliconisation or whether the syringe is a polymer or glass-based system.
Overall, the number of drug products requiring storage in temperatures lower than -20°C has risen significantly in the past few years. There are many reasons for using freezing conditions, such as slowing degradation, extending shelf life and reducing leachables from the container closure.
The growing trend of mRNA applications, especially vaccines, in the advanced therapeutics biologics space is moving to PFSs, requiring a plunger that can function at temperatures down to -50°C. Selected PFS FluroTec® plunger designs meet these criteria and are compatible with ISO glass syringes – as well as some cyclo-olefin copolymer (COC) syringes – that are currently available in the market.
CHALLENGES OF LOW-TEMPERATURE STORAGE
There are challenges for the plunger components in terms of low-temperature storage. The syringes and plungers were designed to fit and function at room temperature. More specifically, they were designed to maintain sterility and CCI, and enable acceptable break loose and extrusion (BLE) forces during administration. It must be determined experimentally whether a PFS intended for use under frozen conditions can achieve an acceptable level of performance. The characterisation of the plunger performance in a PFS is essential to determine the suitability for intended use at low temperature.
The following sections give insights from observations obtained from a performance study conducted with glass and plastic PFSs in combination with FluroTec plungers using a well understood bromobutyl rubber formulation, 4023/50 grey, during and after freeze-thaw exposure.
The FluroTec barrier film lamination used for these respective plungers is a widely proven ethylene tetrafluoroethylene (ETFE) film, which acts as a barrier that reduces interactions between a drug product and the elastomeric plunger and limits the migration of potential leachables from the plunger into the drug product, thereby reducing potential contaminants that may impact drug efficacy and safety. The challenges of storage and delivery of a covid-19 vaccine in a PFS are amplified, as vaccine contact time may be longer with a plunger than with a stopper.
“As pharmaceutical companies seek to move from vials to PFSs for mRNA vaccines, there is a lack of data demonstrating performance and CCI at low temperatures, including -50°C.”
As pharmaceutical companies seek to move from vials to PFSs for mRNA vaccines, there is a lack of data demonstrating performance and CCI at low temperatures, including -50°C. Additionally, there is no standard method for testing CCI on PFSs. All of this makes it difficult for mRNA drug developers to choose and validate proper PFS components for their drugs.
West conducted a study with two FluroTec plunger designs, the 1 mL long and the 1–3 mL. The evaluation was conducted with 1 mL long glass and polymer syringes, and with 2.25 mL glass syringes, respectively. West has developed methods for testing plunger movement, BLE and CCI for syringes that undergo cold storage processing. The BLE method is validated, while the plunger movement and CCI methods were developed by West.
Plunger Movement During Storage at Low Temperature
The PFS system might be affected by pressure change, such as during air transport or low temperature and freeze/thaw cycles. When a drug product freezes, the liquid expands and, depending on the headspace, that expansion can physically push the plunger distally into the non-sterile area of the barrel. When the syringe is brought back to room temperature, foreign material could be introduced into the sterile area, creating a potential risk of contaminating the drug product. Furthermore, moving the plunger from its original position can potentially alter the initial expected break-loose value of the syringe system.
Experimental evaluation was made of water-filled PFSs comprising ISO-standard 1 mL long and 2.25 mL glass staked-needle syringes with FluroTec plungers. Storage conditions were at -50°C (passive freezing in mechanical freezer, vis-à-vis controlled-rate freezing) for >24 hours followed by bench-top thaw for >1 hour. In this experiment, the plunger position was recorded before freezer placement, immediately after removal and after thaw. Emphasis was on movement past the sterile barrier as defined by the sealing ribs.
Container Closure Integrity
CCI plays a crucial role in maintaining the stability and sterility of parenteral products throughout their shelf life. CCI may also be affected by extremely low temperatures, such as -80°C due to a loss of elasticity in the rubber components and the varying shrinkage rates of different materials. For example, it is not uncommon to see that the syringe barrel shrinks less than the plunger, which could result in minimal interference and a breach in the barrier against microbial ingress. In this case, CCI was determined by laser-based headspace analysis for oxygen by comparing values as assembled and after freezer placement and thaw. The test was conducted with 1 mL long-glass and COC barrels paired with a FluroTec long plunger and 2.25 mL glass barrels paired with a FluroTec laminated 1–3 mL plunger. Syringes were divided into three groups: room temperature (RT), -40°C and -50°C storage conditions.
Break Loose and Extrusion Performance
BLE forces were measured by determining the force needed (with tensile testing equipment, e.g. by Instron) to initiate plunger movement and sustain a desired plunger movement rate. A comparison was made between samples before and after freezer placement and thaw. The test was conducted with 1 mL long glass and COC barrels paired with FluroTec laminated 1 mL long plungers and 2.25 mL glass barrels paired with FluroTec laminated 1–3 mL plungers. Syringes were divided into two groups: RT and -50°C storage conditions. The BLE analysis was performed according to ISO 11040-8, section 6.2 with a speed of 100 mm/min.
PERFORMANCE STUDY RESULTS
Plunger movement triggered by lower temperature largely depends on the fill volume. The data generated show that smaller fill volumes result in less movement. In the study, for the 1 mL long syringe filled at 0.5 mL, movement never exceeded 1.5 mm, while, for the syringes filled to 1 mL, movement reached a 3.5 mm distance. The sterile barrier zone for the plungers used in this study was estimated based on drawings at RT in an uncompressed state, and was determined to be around 4 mm. Therefore, to minimise risk of breaching the sterile barrier, not filling syringes to their maximum capacity may be a potential approach. The headspace, material of the barrel, design of the plunger and orientation of the storage used in this study do not seem to affect plunger movement in a significant way. It should be noted that the headspace influences plunger movement in plungers shipped by air transport and can create an additive effect to the movement observed, which is outside the scope of this report. An example of the results is shown in Figure 1 for the 1 mL long glass syringe with two filling volumes.
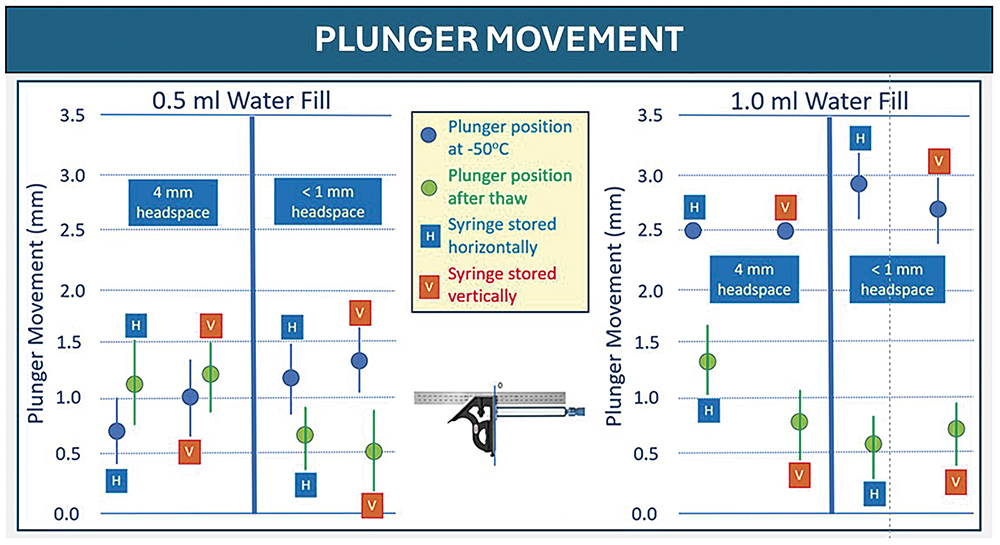
Figure 1: 1 mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw. The sterile barrier is not passed.
CCI testing showed no gross gas exchange during the -40°C and -50°C freezing conditions. Gross exchange is defined as a ≥2% increase in oxygen concentration, which was experimentally determined. The selected polymer system did show a >2% exchange when stored at RT in three out of the 10 syringes tested, which could be expected based on the inherent permeability of COCs.
When stored at cold temperatures, the exchange rates were significantly less, and no values were seen that were >2%. These results indicate that the assemblies of the 1 mL long FluroTec plungers in a standard 1 mL long glass syringe and a standard 1 mL polymer syringe, as well as the 1–3 mL FluroTec plungers in a standard 2.25 mL glass syringe, exhibited promising behaviour that warrants further study for container closure at low temperatures. The evaluation of CCI demonstrated no breach of any PFS. Example data are shown in Figure 2; the oxygen concentration was measured before and after freezer placement and thaw. No increase in oxygen concentration was observed. A breach would result in an oxygen concentration the same as freezer air (i.e. 21%), especially as the decrease in pressure within the PFS upon freezer placement would promote air ingress.
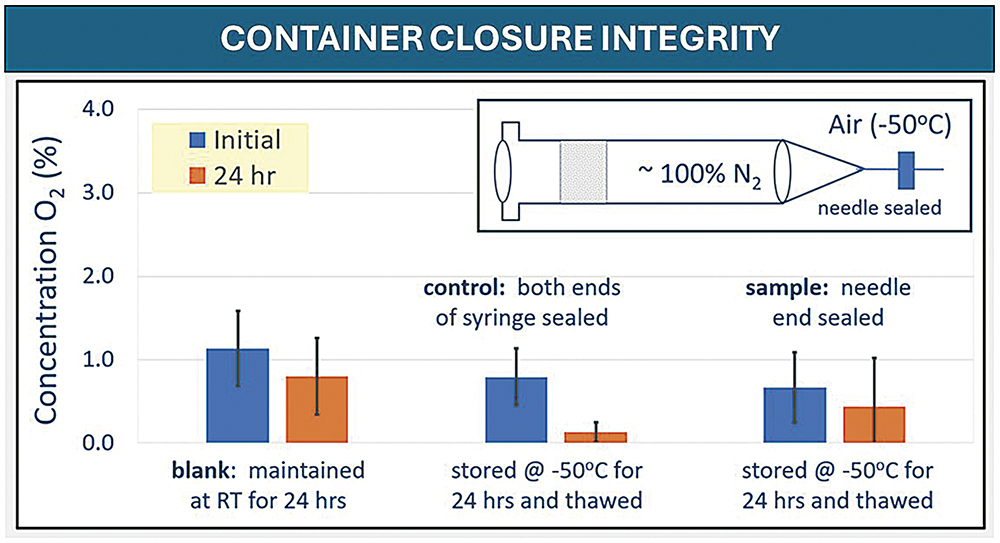
Figure 2: 1 mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw.
“Overall, the evaluation of initiating and sustaining forces at 100 mm/min demonstrated no change in performance for any PFS after freezer placement and thaw.”
The syringes tested for BLE post -50°C storage showed similar appearances. Overall, the evaluation of initiating and sustaining forces at 100 mm/min demonstrated no change in performance for any PFS after freezer placement and thaw. Syringes with FluroTec laminated plungers were tested for BLE forces post -50°C. Both glass syringes sizes showed typical BLE values and performance. The 1 mL polymer syringe showed a slightly higher break-loose, maximum extrusion and average extrusion than the glass syringes, but was still comparable with the glass performance. Example data are shown in Figure 3.
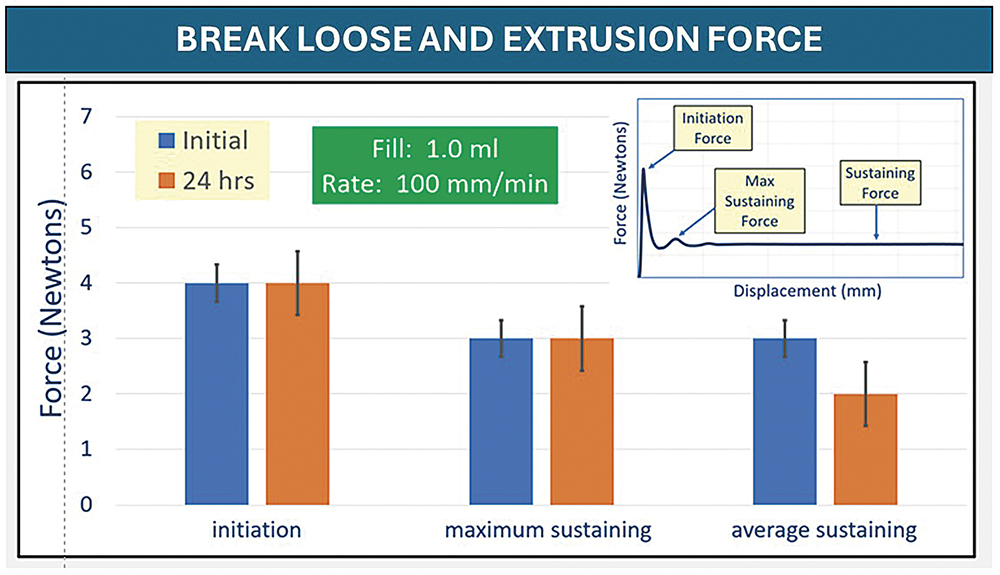
Figure 3: 1 mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw. The sterile barrier is not passed.
It should be noted that all the tests performed were intended to demonstrate examples of the developed tests and not necessarily real-life situations. As noted in the experimental section, test method elements – such as models and materials of the barrels, plungers and plunger rods, plunger placement method and headspace, fill media and volume, storage temperate and other conditions – should be selected based on the intended use. Additionally, other parameters not tested in this work might need investigation, such as air transportation simulations, particle analysis and material properties to determine suitability of the prefilled packaging system for cold storage. Overall, it can be concluded that these testing approaches appear generally suitable for evaluating PFSs systems at low temperatures.
FluroTec is a trademark of West Pharmaceutical Services, Inc. in the United States and other jurisdictions, and FluroTec technology is licensed from Daikyo Seiko, Ltd.
BIBLIOGRAPHY
- Gonzalez M, “From Vial to Prefilled Syringe: Migrating a Drug Product Presentation”. ONdrugDelivery Issue 129 (Feb 2022).
- “Implications of transitioning mRNA Covid-19 vaccines from vial to prefilled syringe”. Schott Pharma, Jun 23, 2022
- McAndrew P, Brasten LJ, Lasinka O, “Performance of Pre-Filled Syringe Systems at Low Temperature”. AAPS PharmSci 360, 2023, Poster Presentation, T1330-09-60.

