To Issue 152
Citation: McCawley M, Lavin A, “Gas-Powered Autoinjector Platform Enables Biologics Drug Delivery”. ONdrugDelivery, Issue 152 (Oct 2023), pp 57–60.
Matt McCawley and Albie Lavin discuss Altaviz‘s universal biologics autoinjector platform, highlighting how its gas-powered drive system, based on Pico-Cylinders, provides numerous benefits over legacy spring-powered autoinjectors.
“Because the gas is contained within the device, drug delivery is effectively silent – there is no loud click or snap like that from the springs found in legacy autoinjectors.”
The AltaVISC universal biologics autoinjector platform is designed to address the performance needs for patient delivery of high viscosities and large dose volumes in the rapidly evolving biotherapeutics market. As large molecule therapeutics drive viscosities into the hundreds, or even thousands, of centipoise range,1 and enzymatic adjuncts2 enable subcutaneous injections well above conventional 2 mL delivery volumes, it is necessary to reinvent the legacy spring-powered autoinjector platform to enable the self-administration of these truly revolutionary therapies. Simply put, these new, game-changing therapies require new, game-changing delivery systems.
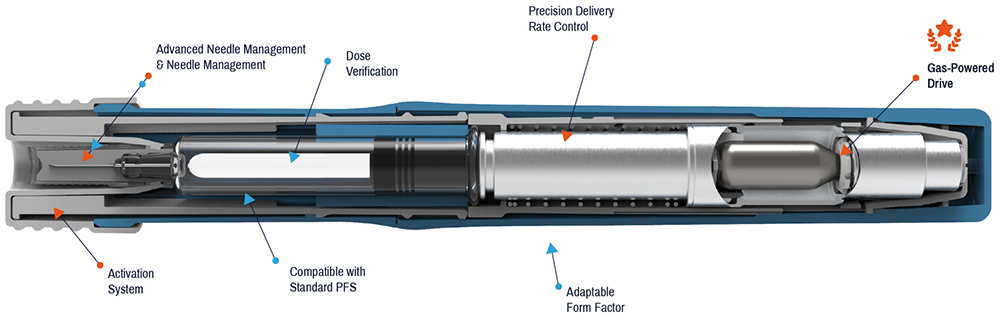
Figure 1: The AltaVISC universal biologics autoinjector platform by Altaviz.
The AltaVISC (Figure 1) is a two-step, low-force activation autoinjector with an actuation mechanism that is configurable to allow buttons, levers, push-on-skin or squeeze triggers. The core drive mechanism is powered by compressed gas cylinders called Pico-Cylinders (Picocyl) that pressurise an expansion chamber, which then drives a plunger into standard size glass syringes, including 1.0 and 2.25 mL. The gas is entirely contained in the expansion behind the plunger and does not make direct contact with the stopper or primary drug container surfaces. Because the gas is contained within the device, drug delivery is effectively silent – there is no loud click or snap like that from the springs found in legacy autoinjectors. Pico-Cylinders can be filled with vapour-phase (N2, Ar) or dual-phase (CO2) gases to a wide pressure range (5–350 bar), allowing plunger force and delivery time to be configurable without requiring spring swaps or housing design changes.
KEY PERFORMANCE ADVANTAGES
The AltaVISC delivers several significant performance advantages over the current state-of-the-art autoinjectors, making the platform suitable for the coming wave of biologic therapies that will require high-viscosity and large-dose delivery.
Soft Start and Low Force Drop-Off Enables Faster, More Consistent Drug Delivery
The AltaVISC exhibits a relatively constant force profile relative to a spring-driven system. This yields a higher average force over the entire actuation stroke, which, in turn, yields a higher average stopper velocity and a reduction in delivery time. Furthermore, there is no large force spike when the device is actuated, as there is with legacy spring-powered autoinjectors, and this “soft start” makes the delivery force curve very consistent.
Higher-Pressure Capacity Enables High-Viscosity Drug Formulation Delivery
The Pico-Cylinders used in the AltaVISC can be filled to pressures up to 350 bar in a very small form factor. This high pressure capacity, along with the faster delivery time enabled by the low force drop-off, allows for delivery of high-viscosity drug formulations. Dose volumes between 0.5 and 2 mL with viscosities up to 100 cP can be delivered through a ½” 27G thin-wall (TW) needle in under eight seconds. The results shown in Figure 2 were calculated using a numerical integration model designed to track plunger motion inside an autoinjector, given a 27G TW needle and a 1.0 mL long and 2.25 mL glass syringe. This model was verified using 100 cP silicone oil in an AltaVISC prototype technology demonstrator.
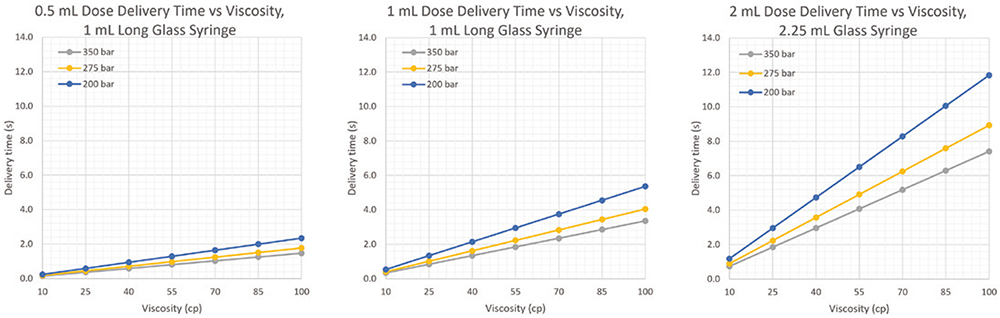
Figure 2: Dose delivery time versus viscosity model data.
Ultra-high viscosity (>1000 cP) drug delivery is also possible with the AltaVISC.3 The numerical integration model data shown in Figure 3 represents different dose delivery time projections using 100–3000 cP formulations through a 25G extra-thin-wall needle.
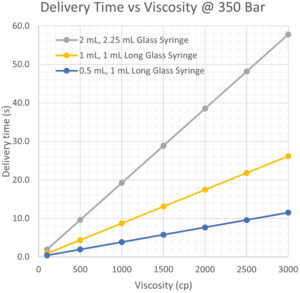
Figure 3: Ultra-high viscosity delivery time model data.
Gas Power Enables Delivery Through Small Gauge Needles
AltaVISC’s combination of high-pressure capacity and faster delivery time enables the use of smaller gauge needles, which improves patient tolerability. The numerical integration model data in Figure 4 shows that 1 mL of 100 cP formulation can be delivered through a 30G TW needle in a 1 mL long glass syringe in less than 15 seconds.
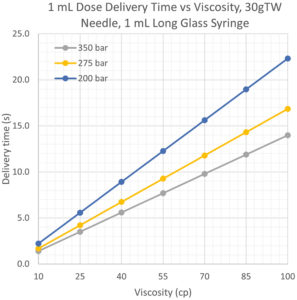
Figure 4: Small needle gauge delivery model data.
Improved Delivery Time Allows for High-Volume Doses
Legacy spring-powered autoinjectors require lower viscosity formulations or larger needle gauges to deliver high-volume doses. The AltaVISC has enough energy to deliver 5 mL through a 27G TW needle in under 30 seconds and could potentially deliver even higher doses if desired (Figure 5).
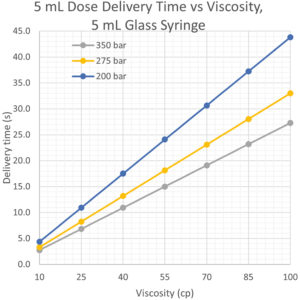
Figure 5: High-volume dose delivery model data.
“This high pressure capacity, along with the faster delivery time enabled by the low force drop-off, allows for delivery of high-viscosity drug formulations.”
PATIENT EXPERIENCE IMPROVEMENTS OVER CURRENT MARKETED AUTOINJECTORS
Altaviz’s AltaVISC uses the same two-step dose delivery workflow as current state-of-the-art autoinjectors, allowing patients and healthcare professionals to keep their current training and use procedures. Additionally, the AltaVISC provides several improvements over the current state-of-the-art autoinjectors with respect to the patient experience. For example, because the AltaVISC uses compressed gas for actuation with a soft start, there is no loud click or snap from a heavy spring extending. Loud noises during actuation can cause significant apprehension in patients when taking their medicine,4 and the silent delivery profile of the AltaVISC allows them to feel more comfortable complying with their dosing regimen.
The AltaVISC also allows for the use of smaller needle sizes than is typical for biologics injections, due to the increased force available from the high-pressure Pico-Cylinder drive. One of the largest complaints patients have about biologics autoinjectors is the pain experienced during injection and extraction of the needle; this pain is directly proportional to needle size. Enabling smaller needle sizes opens the door to products that reduce the pain experienced by the patient, thereby reducing anxiety around taking medication and improving dosing compliance.
RELIABILITY BY DESIGN
Legacy spring-powered autoinjectors are the standard for delivering small molecule therapies in the 1–10 cP range, but quickly reach their limits when applied to high-viscosity and large-volume injections. In typical autoinjectors, the plungers are powered by the potential energy stored in a preloaded stainless steel spring. At time of use, the spring is released and the potential energy is converted to kinetic energy that drives the autoinjector plunger to deliver the drug. In most autoinjectors, there is an intentional gap between the plunger and the stopper to accommodate drug fill tolerances, bubbles and volume changes due to temperature excursions during filling, transportation and final use.5
“The Soft Start feature of gas power now allows the safe delivery of higher viscosities and larger dose volumes in standard glass prefilled syringes.”
Upon the release of the spring, the spring and plunger accelerate until the plunger impacts the stopper, and an impulse force (Figure 6) is created as the velocity of the moving spring mass is rapidly decelerated. This impulse force is significantly higher than the force required to deliver the drug and can contribute to syringe flange breakage. The impulse force also creates increased pressure in the syringe that can lead to syringe container failures. Both failure modes are well known in the industry.3
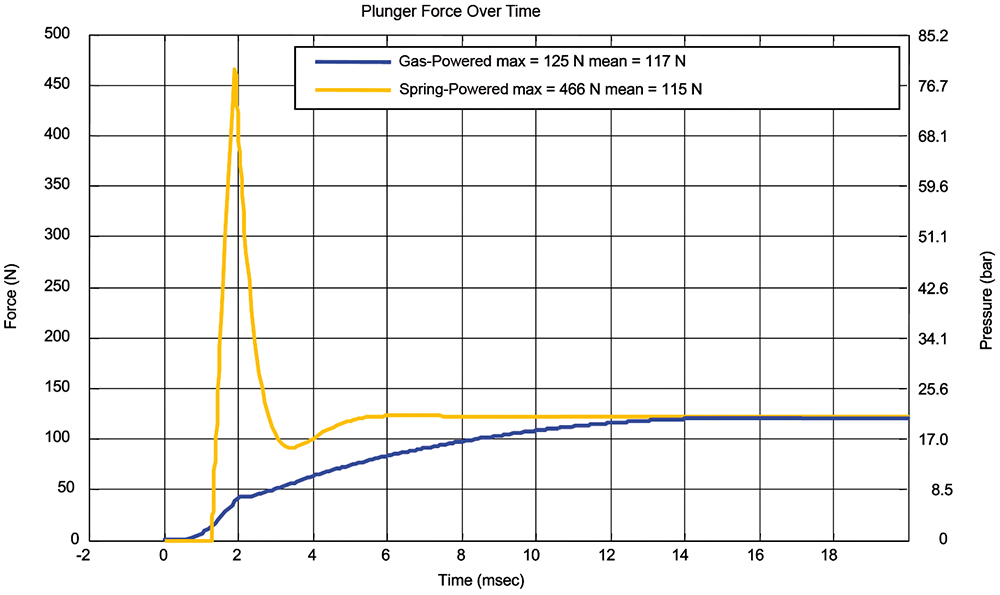
Figure 6: Force and pressure comparison between gas- and spring-powered autoinjectors.
Higher viscosity and larger dose volumes require higher spring forces to administer these drugs in a patient-acceptable delivery time. With higher spring forces come higher spring mass, the impact forces are exacerbated and the probability of syringe breakage increases. A dynamic model was created in MATLAB/Simulink® to simulate the AltaVISC delivering 100 cP silicone fluid from a 2.25 mL glass syringe using a staked 27G TW ½” needle in 10 seconds. There is an initial gap of 5 mm between the plunger and the stopper.
In contrast, the AltaVISC plunger is driven by gas pressure only, eliminating the high mass drive spring. The plunger rod is initially biased so that it comes in full contact with the stopper to eliminate any gap between the plunger and the stopper. Eliminating the gap eliminates the force spike and resulting pressure spike, so the peak force of the system is limited to the delivery force. The force and pressure comparisons are shown in Figure 6.
“Configuring the device to output more or less force for different viscosity formulations or different dose volumes does not require any change to the device mechanism or components.”
The results for the spring-powered autoinjector show a peak force of 466 N, with a 115 N mean delivery force. The peak pressure is 79.4 bar. In contrast, the AltaVISC does not exhibit a peak force spike. Rather, the force ramps up to a mean delivery force of 115 N. The peak pressure is 21.3 bar. As glass syringe breakage is the direct result of the peak force for flange breakage and peak pressure for container rupture, the glass syringe in the AltaVISC would only experience 27% of force and pressure compared with the spring-powered autoinjector, significantly increasing reliability.
The Soft Start feature of gas power now allows the safe delivery of higher viscosities and larger dose volumes in standard glass prefilled syringes. The safety margin taken up by the peak force spike in spring-powered autoinjectors can be converted to useful delivery force, meaning the AltaVISC could potentially provide three times the delivery force of a spring-powered autoinjector while maintaining current standards for reliability.
In addition to designing out primary drug container breakage, the Pico-Cylinder drive system in the AltaVISC has shelf life and shelf stability benefits over spring-powered systems. Pico-cylinders can maintain pressure for over five years, much longer than the shelf stability of typical drug formulations. Typical spring-powered systems require a very heavy spring to be loaded, which runs the risk of stress relaxation in the spring and plastic creep in the polymer components. The performance of spring-powered systems will consistently degrade over the shelf life of the product. The pico-cylinder drive system in the AltaVISC is not loaded during storage, so there is no risk of creep or device integrity loss over a shelf cycle.
SUSTAINABILITY
The AltaVISC can provide a smaller carbon footprint than alternative autoinjector technologies. The Pico-Cylinders that drive the AltaVISC use inert atmospheric gases, such as N2, Ar or CO2, that can be sustainably sourced and managed. They provide a solution that complies with the Kigali Amendment (2019) of the Montreal Protocol on substances that deplete the ozone layer, unlike many gasses that are widely used as propellants in the cosmetics and pharmaceuticals industries.6 Furthermore, the activation spring, cylinder and deep-drawn stamped components that comprise the Pico-Cylinder drive system used in the AltaVISC require 66% less stainless steel compared with typical drive springs used in legacy autoinjectors (15 g vs 45 g).
PLATFORM FLEXIBILITY
The AltaVISC also provides unparalleled flexibility in the product development and manufacturing process.
Same Gas Cylinder Form Factor Delivers 5–350 bar
The Pico-Cylinder used in the AltaVISC can be filled with various gases to a wide range of pressures without requiring a size or form factor change. This means that configuring the device to output more or less force for different viscosity formulations or different dose volumes does not require any change to the device mechanism or components. The same component manufacturing equipment is used to fill the pico-cylinder from 5 to 350 bar.
Accommodates all Drug Formulations
The AltaVISC can be tuned to deliver any drug formulation, including high-viscosity biologics and shear-sensitive molecules. The high-pressure capacity of the Pico-Cylinder and the soft start force profile enable significantly higher delivery forces, in turn enabling short delivery times for high-viscosity drug formulations. For shear-sensitive molecules, the gas pressure inside the Pico-Cylinder can be reduced, and the combination of the soft start force profile and the low force drop-off enables smooth delivery without any potentially harmful pressure fluctuations.
No Loaded Spring During Component Handling and Assembly
The Pico-Cylinder that drives the drug delivery mechanism requires a septum to be opened to release the gas and, prior to actuation, the cylinder is in a completely unloaded state. This means all the components in the device are not experiencing any load during their shelf life or the assembly process, and device assembly is a very simple snap-in operation shown in Figure 7.
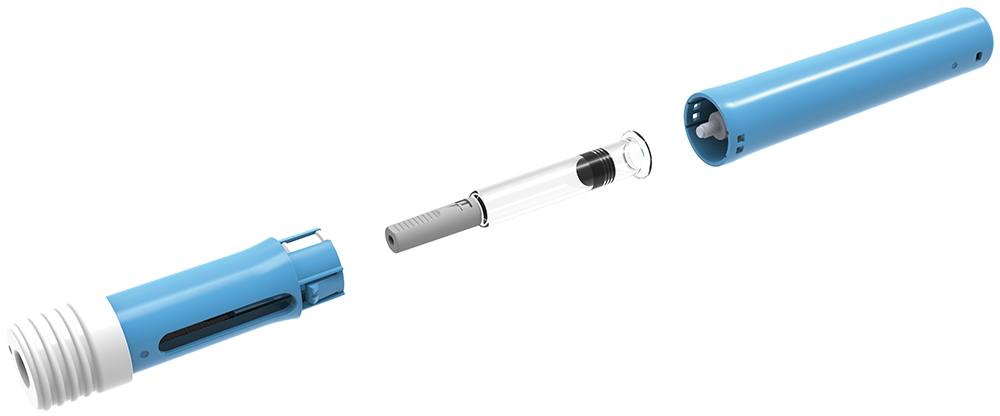
Figure 7: AltaVISC assembly rendering.
Many Gas Options
The Pico-Cylinder used in the AltaVISC can be filled with vapour-phase or dual-phase gases. Vapour-phase gases, such as Ar or N2 exhibit excellent pressure consistency across the typical use case temperature range of 0–40°C, ensuring consistent delivery rates for drug products intended for community use. Dual-phase gases, such as CO2, allow for extremely high expansion ratios, and thus enable larger volumes to be achieved in cases where temperature is more consistent, such as in a clinic or operating room.
Human Factors Experience is Equivalent Across Products
Since the same Pico-Cylinder can be used to deliver different dose volumes or viscosities, the user actuation force and user workflow does not change across different products. Therefore, the human factors development risk is minimal when bringing new products to market.
CONCLUSION
The AltaVISC (Figure 8) elegantly harnesses the high energy density of compressed gas to deliver significantly higher viscosities and larger dose volumes in a patient friendly, reliable, safe, sustainable and flexible platform that is easily adaptable to the current and future needs of the biologics market.
Figure 8: AltaVISC advantages.
To find out more about AltaVISC, visit: www.altaviz.com/altavisc.
REFERENCES
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- “ENHANZE® Drug Delivery Technology: Inspiring New Possibilities”. Company Web Page, Halozyme, accessed Sep 2023.
- “ArQ® Subcutaneous Platform”. Company Web Page, Oval Medical Technologies, accessed Sep 2023.
- Andersen CH, “Giving Yourself Biologic Injections: 23 Practical Tips to Try”. CreakyJoints, Apr 2019.
- Philippson J, Kemp T, “The Interface Between Prefilled Syringe and Autoinjector – A Development Framework”. ONdrugDelivery, Issue 101 (Oct 2019), pp 14–17.
- “About Montreal Protocol”. UN Environment Programme webpage, accessed Sep 2023.

