To Issue 169
Citation: Davidson Z, “Glaucoma in View: Improving Patient Adherence with Multidose Eyedroppers for Preservative-Free Solutions”. ONdrugDelivery, Issue 169 (Mar 2025), pp 30–34.
Zoë Davidson considers the impact of glaucoma on global health and the challenges presented by its increasing prevalence in the ageing global population, discussing how Novelia®, Nemera’s multidose eyedropper for preservative-free formulations, is well-positioned to address many of the challenges presented by glaucoma treatments, from ease of use to sustainability.
INCREASING PUBLIC AWARENESS AND EDUCATION AROUND GLAUCOMA
Glaucoma Awareness Day, observed annually on March 12, serves as a vital reminder of the importance of the early detection and treatment of glaucoma, a leading cause of irreversible blindness worldwide. This day aims to educate the public about the silent progression of glaucoma, which often presents no early symptoms, making regular eye exams crucial for early diagnosis. By raising awareness, Glaucoma Awareness Day encourages individuals, especially those at higher risk, to prioritise their eye health and seek timely medical advice.1
GLAUCOMA MARKET GROWTH PROPELLED BY AN AGEING DEMOGRAPHIC
The growing global ophthalmic eyedroppers market is expected to be driven by the increasing prevalence of eye disease and disorders such as glaucoma, dry eye disease and age-related macular degeneration. Moreover, the increasing ageing population further propels market growth due to a higher incidence of age-related eye conditions.2
Glaucoma represents a group of optic neuropathies characterised by the progressive degeneration of retinal ganglion cells (RGCs). Open-angle glaucoma (OAG) is the most common form of glaucoma, carrying a chronic prognosis and making up 75–95% of primary cases.3 Angle-closure glaucoma (ACG) can either be acute or chronic, but typically has a faster progression than OAG and therefore requires more drastic interventions. These two main types often require the use of eyedrops to reduce intraocular pressure and prevent further damage.
As the population ages, the demand for glaucoma medications administered via eyedroppers is also expected to increase, thereby driving market growth. For example, people are six times more likely to develop glaucoma after the age of 60.4 Given the growing ophthalmic eyedroppers market, drug delivery device designers and manufacturers must strive to not only understand but also provide solutions for patients encountering challenges administering their medication and to improve at-home eyecare management.
A SAFE PRESERVATIVE-FREE SOLUTION FOR PATIENTS
The majority of eye drops today contain preservatives to maintain the sterility of the eye drop formulation. The most commonly used preservative is benzalkonium chloride (BAK), which has been known to damage the cornea over long-term use. Despite consistent data confirming its potential toxic effects, especially for chronic use such as for glaucoma, BAK is still used as the main preservative in eye drops.5
Glaucoma patients have set regimens as prescribed by their healthcare provider and commonly have to fit this regimen into their morning/nightly routine. However, some patients may also use over-the-counter (OTC) dry-eye eye drops in addition, as irritation can be a side effect of some glaucoma medications. According to a 2008 survey conducted in Germany, over 50% of all glaucoma patients have dry eye. Furthermore, dry eye was found to be more common if more than three or more anti-glaucoma agents were used, suggesting that preservatives have an influence on the development of dry eye.6
“THERE HAS BEEN A LOT OF EFFORT TO IMPROVE THE PRIMARY CONTAINER CLOSURE SYSTEMS OF TOPICAL OPHTHALMIC PRODUCTS – FOR EXAMPLE, MULTIDOSE BOTTLES HAVE BEEN IMPROVED TO MDPF BOTTLES.”
There has been a lot of effort to improve the primary container closure systems of topical ophthalmic products – for example, multidose bottles have been improved to multidose preservative-free (MDPF) bottles. As science and technology evolves, there is a greater need to further understand and “preserve” the complex organ that is the eye7 and to ensure that the patient is put first.
To prevent the entry of bacteria into the bottle and/or to filter air, more than half of the bottles designed for multidose preservative-free eye drops on the market rely on a filtering system, with 0.22 μm sterile mesh filters being the industry standard. Significant research has been carried out that challenges their effectiveness.8 Due to their porous structure, bacterial filters do not provide a continuous barrier to contamination.
Nemera’s alternative to the use of sterile filters for multidose eye droppers for preservative-free formulations is a non-return valve system used in conjunction with a silicone membrane to process the returning air (Figure 1). The non-return valve ensures that no liquid can be re-introduced to the container after the drop has been dispensed, completely removing the need to filter the liquid. The intake of air into the dispenser takes place via a separate venting system with a silicone membrane called the PureFlow™. The silicone membrane is a solid, non-porous (unlike bacterial filters) material. It is homogenous and does not contain any holes, meaning that its characteristics can be precisely engineered.
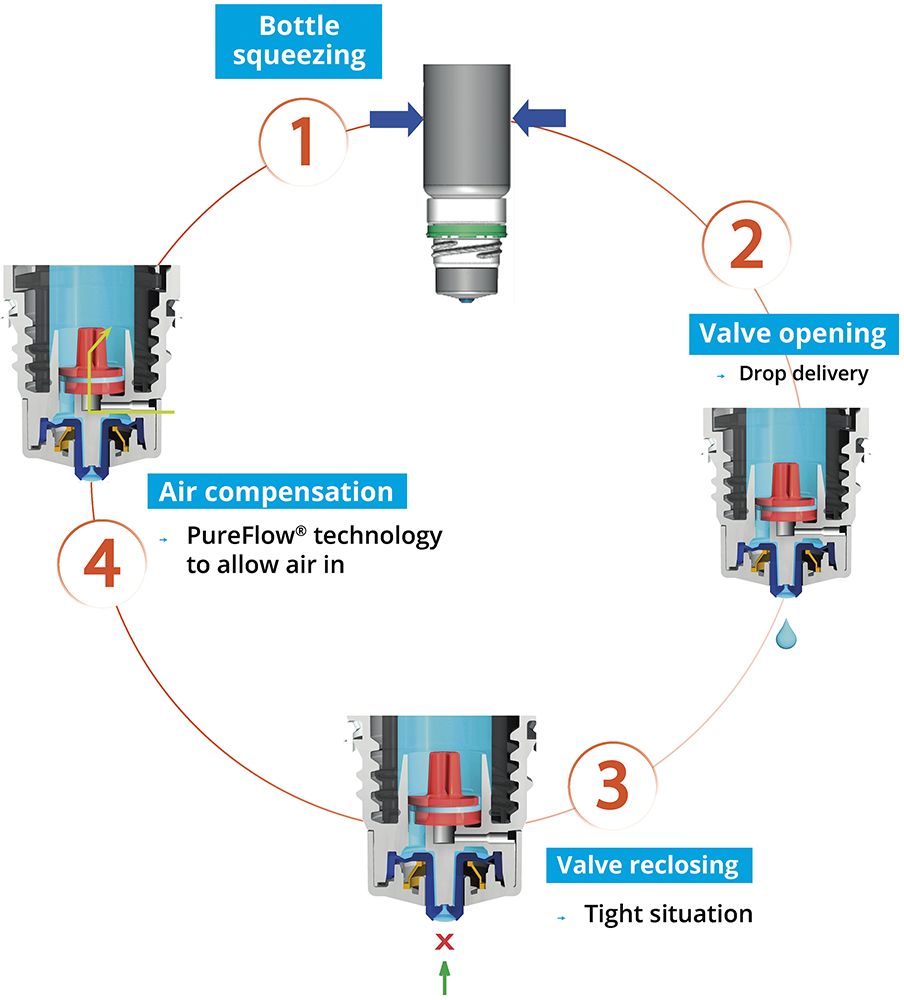
Figure 1: The Novelia system uses a non-return valve that removes the need to filter the liquid.
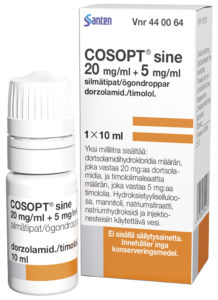
Figure 2: Cosopt iMulti® for the treatment
of intraocular pressure in patients with
OAG using Novelia, marketed by Santen
(image courtesy Santen).
Novelia® has the largest amount of published information regarding the safety and sterility of these MDPF packages and is able to withstand both the likely microbial challenges in real-world scenarios and the more significant and severe challenges that can occur (Figure 2).9
PATIENT CHALLENGES FROM THE HEALTHCARE PROFESSIONAL’S PERSPECTIVE
Self-administration can be challenging for patients with arthritis, tremors and/or hand strength/dexterity issues that can make removing the cap, gripping and squeezing the bottle and holding the hand steady during administration challenging. Additionally, as described by physicians, there is a subset of patients who don’t do well putting things into their eyes. These patients may require aiming techniques and ways to hold the eyelid steady (to avoid blinking). In a worst-case scenario, these limitations may preclude self-administration, requiring a caregiver.
Compliance is often recognised as a challenge by healthcare professionals (HCPs), especially with more complex treatment regimens. Nearly nine out of ten glaucoma patients are unable to instil eye drops correctly and therefore an easy-to-use system that is appreciated by patients could contribute to improving their compliance with their treatment.10 In a study conducted by Nemera’s Insight Innovation Centre, one participant (an ophthalmologist) commented that when you have one drop, compliance is 75–80%, when you use two drops, it drops to 65%, and if you need three drops, it drops below 50%. Additionally, patients (especially those with cognitive issues) may not always remember or follow storage and administration instructions, such as refrigerating the medication or shaking before use.
PRECISION MATTERS FOR PATIENT-CONTROLLED EYE DROP DELIVERY
Patients and HCPs alike desire a bottle that allows patients to control the number of drops delivered, consistently delivering a single drop with each actuation. A study of glaucoma patients found that 90% used an erroneous technique, with many patients missing the eye entirely.11
Novelia’s patented PureFlow technology not only serves as a venting system but also controls the medication flow. Nemera has adapted the flow control within Novelia to avoid delivery of multiple drops into the eye and ensures that only one calibrated drop is dispensed at a time. Nemera offers three different PureFlow versions, each tailored to formulations of differing viscosities, from highly liquid to highly viscous. In addition, five different valve sizes are available, each one delivering a different calibrated drop size. This allows Nemera’s team to customise the drop size depending on specific product requirements. This improved control leads to increased patient confidence that they are delivering an accurate dose, reducing frustration and medication waste.
Patients, even those with dexterity issues and tremors/shaking, must be able to effectively handle/manipulate the delivery system and administer a drop. According to a study conducted for Nemera by GfK (Nuremberg, Germany) in 2015, contributing factors to Novelia being the multidose eyedropper for preservative-free formulations preferred by 76% of patients included the intuitiveness of the screw-on cap and the associated reassurance, as well as the squeeze force required towards the end of the product’s life. Novelia required only 6% more pressure to squeeze the bottle from the beginning to the end of the treatment, compared with 35% for the other devices. Novelia’s patented blue tip is also a favourite feature of the device, as it helps patients target the eye before drop administration and anticipate the angle of the drop on to the ocular surface.
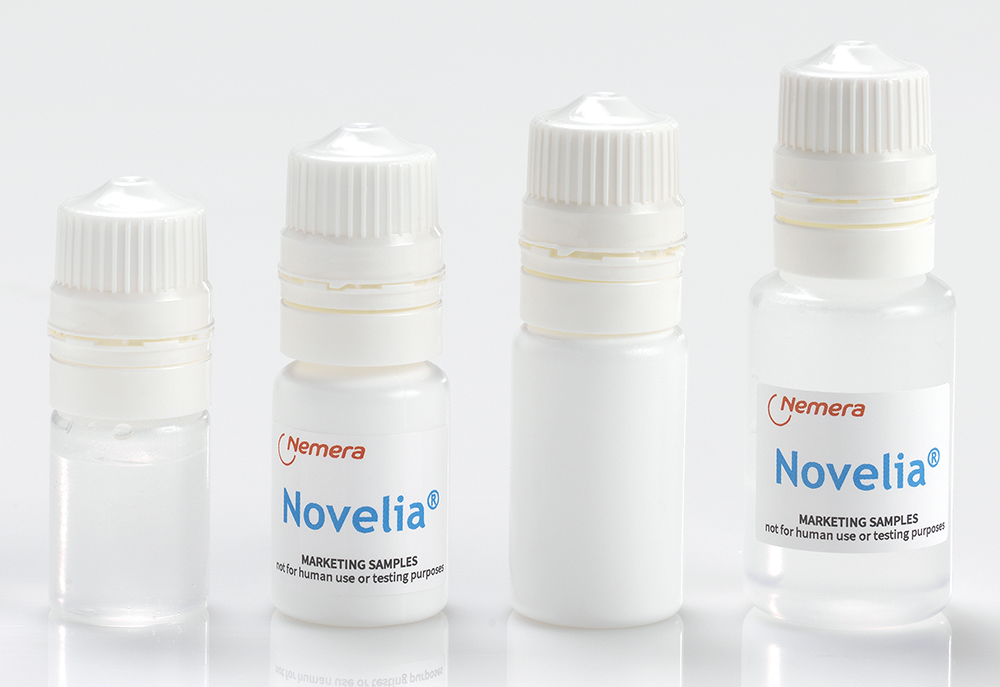
Figure 3: A full range of bottles is available in LDPE and PP in 5 mL, 7.5 mL, 11 mL and 15 mL formats.

Figure 4: Nemera
has recently commercialised a new vented cap version
as part of the Novelia
platform.
A full range of bottles is available in low-density polyethylene (LDPE) and polypropylene (PP), in 5 mL, 7.5 mL, 11 mL and 15 mL formats (Figure 3). Novelia has been validated using both gamma and ethylene oxide (EtO) sterilisation. Offering two options for sterilisation allows Nemera to better meet customers’ compatibility needs. Nemera can also develop additional coloured Novelia caps for specific demands. Finally, Nemera has recently commercialised a new vented cap version as part of the Novelia, aiming to combat challenging formulations and target selected highly regulated markets, thus expanding the scope of ophthalmic treatments served by the MDPF system (Figure 4).
SUSTAINABILITY IN FOCUS FOR TOPICAL OPHTHALMIC APPLICATIONS
When considering unit dose eyedroppers, patients have reported concerns that include cost, as more packaging and eye drop solution is required per dose; waste; and convenience, as it is easier to store a multiuse bottle in a preferred location than it is for a patient to ensure that they have the correct number of unit dose pipettes with them every day.12 Difficult handling has also been pointed out regarding unit doses and their use by elderly patients – inappropriate finger manipulation could be associated with an increased risk of contamination.9 Due in part to the rapidly ageing population, the number of people with glaucoma is expected to increase to over 111 million worldwide by 2040.13
“WITH NOVELIA, THERE IS EIGHT TIMES LESS PLASTIC USED, 25 TIMES LESS DRUG WASTE AND NINE TIMES LESS ENERGY NEEDED FOR TRANSPORTATION COMPARED TO A UNIT DOSE REGIMEN.”
A comparison analysis was conducted by Nemera comparing Novelia with unit-dose packaging for a glaucoma-type regimen (one drop per eye twice per day) over a one-month treatment. The results conveyed that, with Novelia, there is eight times less plastic used, 25 times less drug waste and nine times less energy needed for transportation compared with a unit-dose regimen.
In 2021, Nemera subscribed to the Science-Based Target initiative (SBTi) to define and develop best practices for carbon reduction. One of Nemera’s first objectives is to reduce Scope 1 and 2 emissions by 90% by 2030 (from a 2019 base year). In addition, since February 1, 2024, Nemera’s manufacturing facility in La Verpillière, France, where Novelia is manufactured, has been certified ISCC Plus. This certification scheme for bio-based, renewable and circular raw materials reinforces Nemera’s implementation of sustainability goals. Furthermore, in November 2024, Nemera was awarded the EcoVadis Gold Medal (Figure 5), marking a significant milestone in the company’s sustainability journey. With an impressive score of 79/100, Nemera has not only improved by 12 points since last year but also ranked in the top 1% of companies in the industry for social and environmental responsibility.

Figure 5: In November 2024, Nemera
was awarded the EcoVadis Gold Medal, marking a significant milestone in the company’s sustainability journey.
ENABLING CUSTOMERS TO SERVE PATIENT NEEDS WORLDWIDE
Nemera offers a range of laboratory services for Novelia, including testing of customers’ bulk formulation. This testing comprises usage simulation over a two-week period, drop size analysis (variable depending on valve diameter), flow control and squeeze force testing (beginning and near end of life). These tests enable Nemera to determine the best Novelia configuration for a particular customer formulation. Nemera can recommend the most suitable PureFlow control, bottle type and valve size to achieve the desired drop calibration.
Nemera’s regulatory team is on hand to support customers with their submission filing, providing guidance on supportive documents for registration. Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling of certain molecules with the Novelia delivery system. Nemera has a substantial list of partners, formulation licensors and fillers, all working in collaboration to bring to customers a finished drug-device combination product with Novelia.
While it’s important that containers be user friendly, it has been found that educational resources instructing patients to apply their eye drops correctly mitigate many issues with unintentional noncompliance.14 Nemera can support customers with product market launch, for example, by educating sales teams and HCPs on the delivery device, offering dedicated training and providing materials to assist in promotional material creation. Customisable patient guidance videos are also available in several languages to increase patient compliance around the world.
Today, Novelia has approximately 400 references on the market for prescription and OTC products in over 60 countries across Europe, Latin America, North America, Oceania, the Middle East and Asia Pacific. To serve customers in supporting patient needs, Nemera is once again increasing its manufacturing capabilities, this time in France. This capacity increase comes shortly after Nemera’s US expansion in 2023, which saw the company double its capacity to produce Novelia® multidose eyedropper for preservative-free formulations.
REFERENCES
- Barden A, “What is World Glaucoma Week/Day?”. All About Vision, Feb 2024.
- “Ophthalmic Eye Dropper Market Size, Share & Trends Analysis Report By Type, By Drug Type, By Treatment Type, By Region, And Segment Forecasts, 2024–2030”. Market Report, Grand View Research, Jan 2024.
- “Glaucoma Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape”. Market Report, GlobalData, Nov 2022.
- Allison K, Patel D, Alabi O, “Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future”. Cureus, 2020, Vol 12(11), art e11686.
- “2007 Report of the International Dry Eye WorkShop (DEWS)”. Ocul Surf, Vol 5(2), pp 65–204.
- Erb C, Gast U, Schremmer D, “German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye”. Graefes Arch Clin Exp Ophthalmol, 2008, Vol 246(11), pp 1593–1601.
- “Ophthalmic Product Development, From Bench to Bedside” (Neervannan S, Kompell UB eds). Springer, 2021.
- Hasegawa H et al, “Membrane filter (pore size, 0.22-0.45 micro m; thickness, 150 micro m) passing-through activity of Pseudomonas aeruginosa and other bacterial species with indigenous infiltration ability”. FEMS Microbiol Lett, 2003, Vol 223(1), pp 41–46.
- Campolo A, Crary M, Shannon P, “A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops”. Biomed J Sci & Tech Res, 2022, Vol 45(1), pp 36035–36044.
- Gupta R et al, “Evaluating eye drop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192.
- Tatham AJ et al, “Eye drop instillation technique in patients with glaucoma”. Eye (Lond), 2013, Vol 27(11), pp 1293–1298.
- Erb C, “Glaucoma and Dry Eye”. Uni-Med Verlag, 2012.
- Tham YC et al, “Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis”. Ophthalmology, 2014, Vol 121(11), pp 2081–2090.
- Davis SA et al, “A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique”. Patient Educ Couns, 2019, Vol 102(5), pp 937–943.

