To Issue 152
Citation: Primavessy D, Saaler-Reinhardt S, Maksymowicz K, Stockton M, “Gx Inbeneo® – Developing an Autoinjector that Responds to the Challenges of Biologics”. ONdrugDelivery, Issue 152 (Oct 2023), pp 32–36.
Daniel Primavessy, Sigrid Saaler-Reinhardt, Katarzyna Maksymowicz and Marie Stockton discuss how the Gx Inbeneo® autoinjector from Gerresheimer was purpose designed to provide an effective, easy-to-use solution for the subcutaneous self-administration of sensitive biopharmaceuticals.
Over the past few years, there has been a significant shift towards the development of biologic drugs due to their effectiveness in treating chronic conditions, such as cancer, rheumatoid arthritis and psoriasis. Since 2017, seven out of the top ten drugs on the market by sales are biopharmaceuticals.1 However, biologic drugs present a number of challenges to effective delivery, including the potential for elevated viscosity with increased concentration, larger volumes, and the sensitive nature of the biomolecules.
In parallel, there is a growing preference for subcutaneous self-administration in an at-home setting within the sector, as it facilitates patient independence and a decrease in cost for the healthcare system. To support effective delivery of biologic drugs, a device therefore needs to overcome the challenges presented by the drug formulation and, at the same time, facilitate autonomous patient usage.
“Rather than using a staked-in needle, as with a PFS, the Gx Inbeneo® design concept uses a double-ended needle that is separated from the primary container. ”
GOING BEYOND THE AUTOINJECTOR STATUS QUO
In the past, the most common delivery method for biologic drugs has been administration in a clinical setting by a healthcare professional, either intravenously or subcutaneously via a prefilled syringe (PFS).2 Transferring to a home-care setting has led to the development of autoinjectors that offer advantages such as easier administration and needlestick injury prevention. The standard approach to autoinjector design for biologics is to encapsulate the primary container of a staked-needle PFS within a device, adding a spring mechanism to apply force to the plunger. Such autoinjectors employ a compressed spring held in place with a locking mechanism that must be reliably released at the moment of injection. The dual role as “force barrier” and release mechanism is a challenge for many autoinjectors when the spring force is increased to deliver elevated volumes, viscosities or both. This has resulted in several incidences of misfiring over the past two decades.3
To overcome this challenge, Midas Pharma and Gerresheimer chose a cartridge-based, pre-pressurised design for the Gx Inbeneo® autoinjector (Figure 1). The autoinjector is based on the NIS autoinjector prototype.4 The unique, patented design employs a robust prefilled glass cartridge to retain the spring force, thus making the primary packaging the force barrier. No additional spring locking mechanism is required, which eliminates the associated risk of failure. This innovation enables the use of springs strong enough to inject drug products with viscosities up to 100 cP at volumes of up to 3 mL (based on calculations).
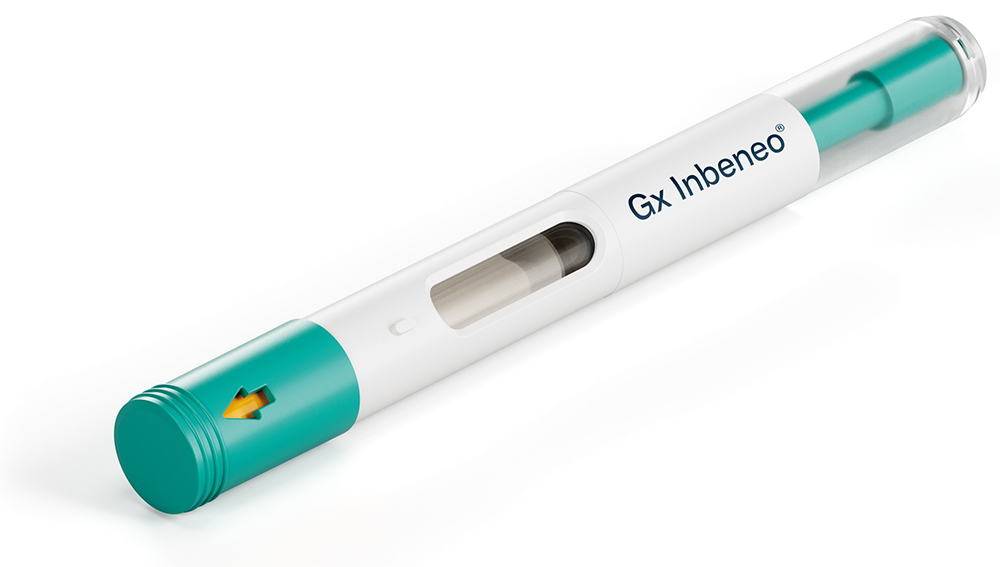
Figure 1: The Gx Inbeneo® autoinjector for biopharmaceuticals.
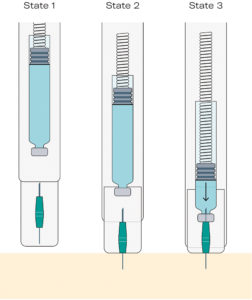
Figure 2: An overview of the injection mechanism of the Gx Inbeneo®, showing how the fluid path is established in a single user step.
Rather than using a staked-in needle, as with a PFS, the Gx Inbeneo® design concept uses a double-ended needle that is separated from the primary container. The needle is held separately in a needle carriage protected by the device’s needle safety shield (Figure 2, State 1). To begin injection, the patient presses the device against the skin, the needle safety shield moves backwards and the front of the needle pierces the skin (Figure 2, State 2). Subsequently the needle carriage moves backwards, and the other end of the needle pierces the septum of the cartridge. The fluid pathway is then established, and the drug is automatically and smoothly injected (Figure 2, State 3). The separation of the needle from the primary packaging until administration and the double-ended design additionally address other challenges of biologic drugs, as detailed in subsequent sections of this article.
PROOF OF CONCEPT FOR THE CARTRIDGE-BASED DESIGN
The concept of using the prefilled cartridge as the force barrier was successfully validated during extensive testing by Midas Pharma and Gerresheimer. ISO-standard cartridges from Gerresheimer and two other suppliers were tested along with septa and plunger stoppers from several different manufacturers. The results demonstrated that the device’s break-loose and glide forces were within acceptable ranges. At maximum pressure, the septa did not rupture when pierced, and piercing forces were also within acceptable ranges. Cartridge septa showed slight bulging for the first 14 days, after which they became stable. The Type I non-hardened pharma-grade glass of the cartridges remained stable at pressures of magnitudes above those employed in the Gx Inbeneo®.
In a second set of tests, cartridges were filled with a commercially available adalimumab formulation and stored under pressure for six months. The drug formulation was expelled through the fluid pathway and then subsequently tested with size exclusion chromatography and gel electrophoresis. No signs of aggregation or protein denaturation were found.
“Three design features of the Gx Inbeneo® autoinjector respond to the risk of aggregation – the separation of the needle from the primary container prior to activation, the use of cartridges with baked-on siliconisation and the pre-pressurised system.”
ADDRESSING THE SENSITIVE NATURE OF BIOLOGIC DRUGS
Sensitive biotherapeutics, such as monoclonal antibodies are vulnerable to aggregation, which can result in a loss of biologic functionality. This can be caused by interaction with substances such as tungsten, glue residues, silicone oil droplets or a gas-liquid interface present as gas bubbles. Three design features of the Gx Inbeneo® autoinjector respond to the risk of aggregation – the separation of the needle from the primary container prior to activation, the use of cartridges with baked-on siliconisation and the pre-pressurised system.
Separated Needle
Unlike with a staked-in needle PFS, there is no contact between the drug product and needle during storage in the Gx Inbeneo® as the needle is separated from the primary packaging. Therefore, the possibility of the drug product entering the needle and causing clogging is eliminated. Furthermore, if there were to be glue or tungsten residues, they could not negatively affect the drug product.5–7
Baked-On Siliconisation
The risk of protein aggregation caused by silicone oil droplets migrating into a sensitive biologic drug is significantly reduced by using a baked-on siliconisation process.8 This process is generally challenging with a staked-in needle PFS, due to potential impact of the required high-temperature on the staked-in needle bonding; however, it can easily be applied to cartridges, such as those used in the Gx Inbeneo®.
Reduced Gas Bubble Presence
An inherent property of the Gx Inbeneo® technology is that any gas bubbles present in the liquid within the cartridge are reduced or dissolved due to the applied pressure, according to Henry’s Law. This minimises gas-liquid interface in the primary container, which limits the potential for protein aggregation in drug products with sensitive biotherapeutics.9
“For sensitive biologics, terminal gas sterilisation with NO2 is superior to the commonly used process with ethylene oxide, as NO2 sterilisation can be carried out at lower temperatures and NO2 ingress has been shown to be negligible.”
STERILISED AND READY-TO-USE
Considering the sensitive nature of biologics is crucial when developing a sterilisation approach for a drug delivery device. To address this topic, Gerresheimer offers two sterilisation options for the Gx Inbeneo® that are compatible with biologic drugs – terminal gas sterilisation with nitrogen dioxide (NO2) and aseptic assembly. For sensitive biologics, terminal gas sterilisation with NO2 is superior to the commonly used process with ethylene oxide, as NO2 sterilisation can be carried out at lower temperatures and NO2 ingress has been shown to be negligible.10 An alternative strategy is aseptic assembly of the device in a cleanroom environment, which avoids the need for terminal gas sterilisation entirely. Regardless of the sterilisation method, the autoinjector is delivered blister-packed, ensuring that it maintains sterility for its entire shelf life and is ready to use as soon as it is needed.
BALANCING INJECTION TIME AND PATIENT EXPERIENCE
Other than employing a spring with a higher force, there are two approaches for administering higher viscosities and larger volumes of a drug – increasing injection time or employing a thicker needle. Both these options come with compromises. A thicker needle is less comfortable for the patient, which negatively impacts the patient experience. Extending the injection time can make it difficult for some patients to hold the autoinjector stable for the full duration of the dose, which creates a risk that the full dose is not delivered, decreasing effectiveness of the treatment.
Rather than accept these compromises, the development team at Gerresheimer and Midas Pharma came up with a solution – a double-ended needle with different thicknesses at each end. The thicker end of the needle pierces the septum of the primary container while the end of the needle that pierces the patient’s skin is thinner. This innovation mitigates the potential for discomfort caused by a thicker needle piercing the patient, while also enabling higher viscosities to be delivered in less time than with a standard needle.
PROVEN USER BENEFITS
As well as being able to deliver biologic drug formulations reliably, the development team at Gerresheimer and Midas Pharma also ensured that the Gx Inbeneo® autoinjector is safe and simple to use, thereby supporting effective delivery of therapy. As previously described, Gx Inbeneo® has significant advantages in terms of limiting potential discomfort and inconvenience when self-administering biologics. The design of the autoinjector also reduces the number of user steps and their complexity, which decreases the potential for use error. For example, there is no need for the patient to attach the needle themselves, as is the case with some other cartridge-based autoinjectors. Additionally, the pre-pressurised system allows for straightforward push-on-skin activation, with no need to press a button.
Further optimisations were made to the design to support the patient during drug administration. Most autoinjectors currently on the market include a viewing window, located in the middle of the device, to inspect the medicinal product and visually track injection progress. When designing the Gx Inbeneo®, it was observed that many patients need to grasp the device with their whole hand – often due to dexterity problems caused by their disease, thereby covering the central window. To overcome this issue, the Gx Inbeneo® has been equipped with a transparent top casing, through which the patient can view a coloured indicator move downwards as the drug is injected.
All of the features relevant for patient administration were tested during a formative usability study. The results of the study were highly positive – the feature to visually track the injection progress via the transparent top casing was very well received and participants were observed to position the device correctly against the body for the full injection time. As a further indication of the end of dose, Gx Inbeneo® makes an audible click at end of dose.
READY FOR A SPECIFIC BIOLOGIC DRUG
With biopharmaceuticals becoming increasingly prevalent on the market, pharmaceutical companies need to select a device that effectively and reliably delivers their sensitive drugs. They are also looking to provide therapeutic support to patients as quickly as possible, making time-to-market another important consideration. With the Gx Inbeneo® autoinjector platform, Gerresheimer and Midas Pharma have drawn on their experience and gathered patient feedback to ensure these requirements have fully informed the device design.
The modular platform concept of the Gx Inbeneo® means that it can be quickly customised to a wide range of drug formulations. Patient needle gauges of 25G, 27G or 29G can be combined with ISO standard cartridges from Gerresheimer or other suppliers with up to a 3 mL fill volume. Spring forces can also be adjusted, allowing for an optimal balance of patient experience and injection time, even with highly viscous biologics (Figure 3). The modular design concept and manufacturing readiness reduces the timeframe for clinical trial and market launch.
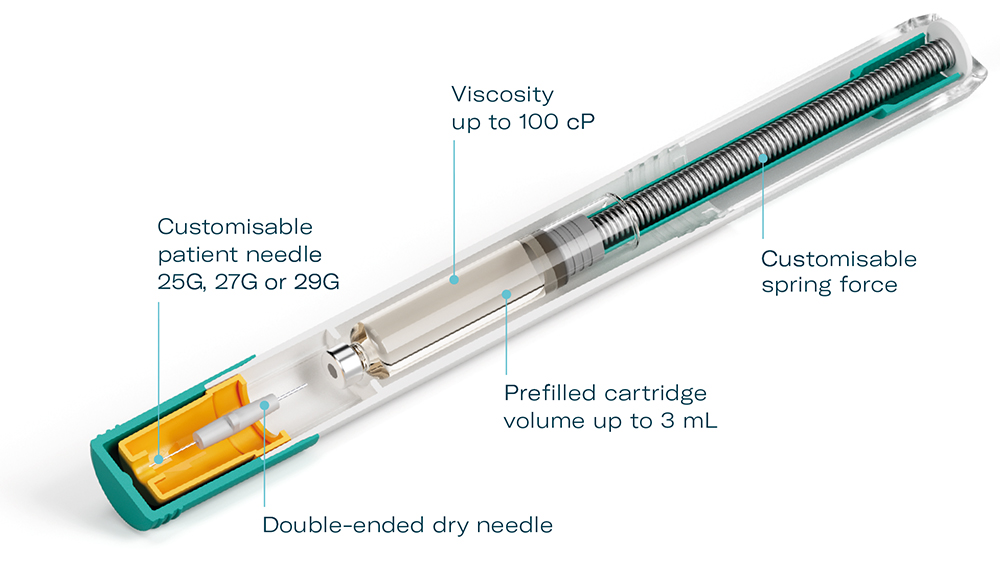
Figure 3: Gx Inbeneo® modular platform concept.
What is more, Gerresheimer is a full-service provider with many years of experience in primary packaging, device development, manufacturing and regulatory submission. All of these factors enable Midas Pharma, Gerresheimer and their pharmaceutical customers to reduce time-to-market for the combination product. Thus, new drug therapies can be provided to patients as quickly as possible to help them manage their health conditions and maintain their quality of life.
Discover more about Gx Inbeneo® by visiting: gerresheimer.com/en/drug-delivery-systems/platform-solutions/autoinjector.
REFERENCES
- Jones GB et al, “Subcutaneous drug delivery: An evolving enterprise”. Sci Transl Med, 2017, Vol 9(405).
- Badkar AV, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- “Simponi (golimumab) 50 mg and 100 mg: important changes to the injection instructions for the SmartJect Pre-filled Pen”. EMA Direct Healthcare Professional Communication, Aug 2023.
- Oakley T, Saaler-Reinhardt S, “Development of a New High-Performance Autoinjector”. ONdrugDelivery, Issue 105 (Feb 2020), pp 14–17.
- Scheler S et al, “Needle clogging of protein solutions in prefilled syringes: A two-stage process with various determinants”. Eur J Pharm Biopharm, 2022, Vol 176, pp 188–198.
- Murphy MI et al, “Effect of Various Silicone Oil And Tungsten Levels on the Stability of a Monoclonal Antibody in Nine Commercially Available Prefilled Syringes”. J Pharm Sci, 2023, Vol 112(6), pp 1586–1594.
- Bee JS et al, “Precipitation of a monoclonal antibody by soluble tungsten”. J Pharm Sci, 2009, Vol 98(9), pp 3290–3301.
- Funke S et al, “Silicone Migration From Baked-on Silicone Layers. Particle Characterization in Placebo and Protein Solutions”. J Pharm Sci, 2016, Vol 105(12), pp 3520–3531.
- Leiske DL, Shieh IC, Tse ML, “A Method To Measure Protein Unfolding at an Air-Liquid Interface”. Langmuir, 2016, Vol 32(39), pp 9930–9937.
- Opie D, Goulet E, “Nitrogen Dioxide” in “Block’s Disinfection, Sterilization, and Preservation” (McDonnell G, Hansen J, eds). Lippincott Williams and Wilkins, 6th Edition, Sep 2020.


