To Issue 128
Citation: Alt A, Pianezzi S, “How Miniaturised Liquid Flow Sensors are Revolutionising Subcutaneous Drug Delivery”. ONdrugDelivery, Issue 128 (Dec 2021), pp 44–47.
Andreas Alt and Susanne Pianezzi describe the drivers accelerating industry trends towards both wearable subcutaneous injection devices and connecting such devices to monitor patient dosing, providing a number of benefits. They go on to explain how Sensirion’s miniaturised disposable liquid flow sensors can confirm and track subcutaneous drug delivery in real time, enable precise dosing in terms of flow-rate as well as administered volume, and enable automatic failure detection, adding substantial value at low cost.
“With the covid-19 pandemic accelerating the push towards digitalisation … smart delivery systems for monitoring how, when and where patients use their therapeutics have really come to the fore.”
Whether smart injectors, infusion pumps or digital pills – digital drug delivery is revolutionising the medical and pharmaceutical industry and accelerating the emergence of self-care. As demographic change leads to an increase in patients with chronic diseases, the need for a larger number of digitalised home care therapies grows.
The increasing demand for self-treatment at home, as well as the rise of biotechnology-based therapeutics and precision medicine, have prompted the rise of wearable subcutaneous injectors. The trend for connecting these and other drug delivery devices to the Internet of Medical Things (IoMT) had already grown strong throughout the 2010s. With the covid-19 pandemic accelerating the push towards digitalisation across the board, and patient-centric solutions in healthcare further, smart delivery systems for monitoring how, when and where patients use their therapeutics have really come to the fore.
Implementing miniaturised liquid flow sensors in wearable devices allows subcutaneous drug delivery to reach the next level, benefitting patients, doctors, nursing staff, pharmaceutical companies and healthcare systems.
ACCELERATING PATIENT-CENTRICITY
“Another group of stakeholders driving the adoption of smart delivery systems is payors such as health insurers.”
The covid-19 pandemic challenged hospitals all over the world. Scheduled surgeries and therapies were put on hold due to a shortage of beds, over-burdened personnel and fear of infection. Patients became wary about visiting doctors’ offices and hospitals, and in some countries were even encouraged by the government to stay away, and so they resorted to telemedicine appointments where possible.
The pandemic shone a light both on shortcomings and areas where smart technology can make a real positive impact, both in terms of being prepared to deal with future healthcare crises such as covid-19 but also more generally. Reducing hospital visits for routine checks and treatments of chronically ill patients is a key part of the required transformation, likewise improving efficiency of medical staff to help reduce overall treatment costs.
Connected digital technology can play an important role by improving collaboration between nurses, doctors, management and patients, providing additional safety by monitoring drug delivery and treatment and allowing more data-driven interpretation of treatment plans and a faster response to changing patient conditions. New portable or wearable designs enable patients to manage their conditions safely at home, with more individualised, flexible treatment options supported by remote monitoring.
From a macro-economic viewpoint, the trend towards digitalisation is associated with decreasing prices of electronic components. This not only justifies more competitive equipment pricing, but also enables medical device manufacturers to innovate and develop new designs incorporating digital elements that truly add value. For example, the direct documentation of ongoing treatments and the accurate logging of the amount and timing of successfully delivered drugs in hospital electronic health records (EHR), could also easily be extended to treatments taken at home.
Another group of stakeholders driving the adoption of smart delivery systems is payors such as health insurers. Pharmaceutical and insurance companies have been moving towards models that incorporate proof of use or even proof of effectiveness.
Smart inhalers that measure the inhaled flow profile and dose actuation, for example, are already able to prove that the dose was taken correctly.
“Sensirion’s sensor solutions allow miniaturised, disposable liquid flow sensors to be integrated into LVIs to control, confirm and track subcutaneous drug delivery in real time. They enable precise dosing in terms of flow-rate as well as administered volume, and enable automatic failure detection.”
BIOPHARMACEUTICALS AS A DRIVING FORCE
Whether for personalised or participatory patient care, precision medicine and advancements in biotechnology also have an impact on self-care trends. Compared with conventional medicines, the use of high-value drugs enables diseases to be treated in a more targeted way with fewer side-effects.
Unlike chemically synthesised drugs, biopharmaceuticals are made of complex structures derived from micro-organisms, mammalian cells or plant extracts. For example, they include proteins that stimulate blood cell formation, insulin, or antibodies that inhibit the growth of cancer cells. These high-value drugs also improve the opportunities to cure other previously untreatable diseases like autoimmune disorders, cardiovascular diseases, diabetes or neurological disorders.
However, because they must be administered parenterally, biopharmaceuticals are still not universally accepted. Due to the large size of the molecules, the most prevalent mode has traditionally been intravenous infusion, which requires professional clinical support, adding clinical costs to already high production costs.
The specialised handling that high-volume and viscous formulations require means that conventional drug delivery devices are not compatible or suitable. Furthermore, some new drugs require specific dose timing, concerning flow rate for example, while others are in a lyophilised state and require reconstitution prior to administration. To overcome these administration-related challenges, new drug delivery mechanisms have been developed, with wearable subcutaneous large-volume injectors (LVIs) being a notable category.
LVIs designed for existing products have strong commercial benefit, creating new revenue streams. For example, the patent for Amgen’s Neulasta (pegfilgrastim) expired in 2015. The new on-body injector version of the product, Onpro, led to an extension of the drug’s product lifecycle.
CONNECTED LARGE-VOLUME INJECTORS
For a few years now, LVIs – also called on-body delivery systems, patch pumps or wearable drug-delivery devices – have been enabling pharma companies to move products from intravenous infusion presentations to subcutaneous injection and with it the opportunity for self-administration at home. In particular, prefilled and preloaded drug-device combinations, as well as those with simple and intuitive patient filling steps, are providing a more convenient yet safe and reliable alternative to outpatient treatment. The patient-filled systems allow lyophilised drugs, that need be delivered by the user shortly after reconstitution, to be filled at the point of use.
Connecting these devices to the IoMT means that they not only enable patients to receive therapeutics at home, but they also enable therapy to be monitored in real time and remotely, increasing adherence, and reducing both effort and cost for patients, healthcare providers and insurers.
Because of the high volumes and viscosities, delivery of the biopharmaceutical dose from the device must be controlled, confirmed and tracked. This requires accurate and reliable sensor technology to be incorporated into the device.
Challenging Device Design
The market for LVIs for non-insulin drugs is expected to grow at a fast pace during the current decade. Over 50 such wearable products and more than 10 drug-device combinations with high storage capacities are already either commercialised or in development. Whether selling devices including a medication or not, the drug-delivery industry is facing many design challenges: improving the handling of viscous formulations and optimising usability, as well as keeping devices small and costs low. Consequently, most LVIs are made of a combination of both disposable and re-usable parts, which makes sense in terms of environmental sustainability.
The battery, motor, readout electronics, connectivity module and display are re-usable, the needle, drug compartment, patch and wetted sensors are disposable.
Miniaturised, Cost-Effective, Disposable Sensors
Since there are various biopharmaceuticals with different properties, LVI designers must individually guarantee reliable, precise function as well as ease of use. Up to now, they equipped devices with visual, audio or tactile indicators for needle positioning and on-body attachment. Even failures like occlusions can be detected to some degree, but currently only in an indirect way, leaving the possibility of false positives.
Even more important is the ability to incorporate direct flow measurement and measure delivered volumes accurately, as well as bidirectional measurement capability, which conventional sensors are not capable of. Sensirion’s sensor solutions allow miniaturised, disposable liquid flow sensors to be integrated into LVIs (Figure 1) to control, confirm and track subcutaneous drug delivery in real time. They enable precise dosing in terms of flow-rate as well as administered volume, and enable automatic failure detection, such as occlusion or air-in-line, in a cost-effective and direct manner.
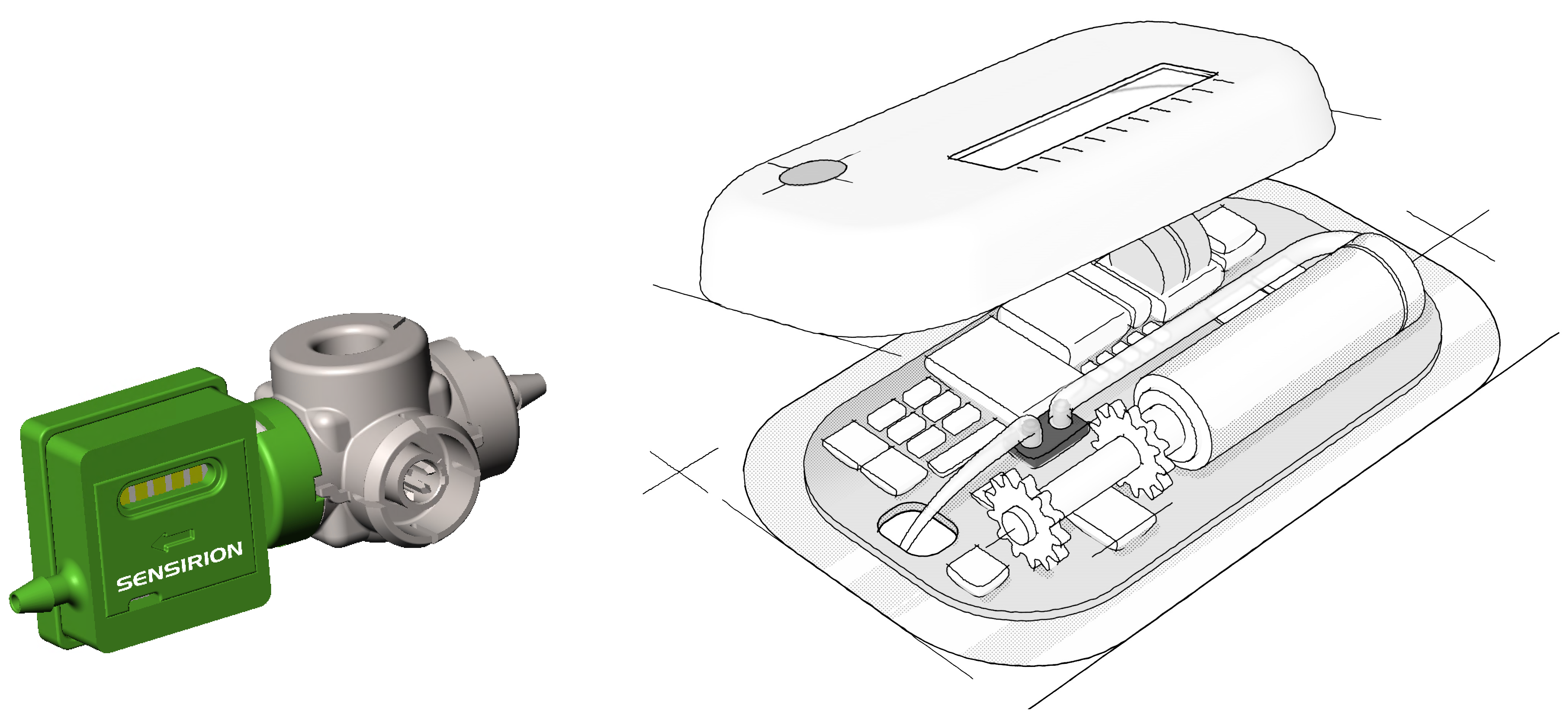
Figure 1: Sensirion’s sensor solutions allow miniaturised, disposable liquid flow sensors (left) to be integrated into large-volume injectors (right). Images not to scale.
Integrated into a connected LVI, these next-generation sensors not only allow the administration to be monitored by the patient via a smartphone app, but additionally enable communication such as telemetry with stakeholders involved in the patient’s care, like family members, parents or relatives. Nursing staff, doctors, pharmaceutical companies (for research) and health insurers (for verification) receive updates as well as metrics about the administration and the device status. Programmable features could also adapt or optimise the subcutaneous drug delivery process.
If an injection device shows issues, a flow sensor can provide peace of mind to patients as well as their relatives. Put simply: implementing a tiny smart sensor improves therapy outcome, patient adherence and quality of life.
Miniaturised liquid flow sensors enable LVIs to:
• measure the flow rate directly and bidirectionally to confirm the administered fluid volume in real time
• monitor system performance and guarantee reliable failure detection
• enable connected solutions for therapy monitoring and tracking by all stakeholders.
Sensor, Pump and Beyond
When designing LVIs, it is recommended to view the liquid flow sensor and the pumping mechanism from a holistic point of view. To identify the ideal design of the flow control system in terms of size, performance, ease of integration, manufacturability and cost, medical device manufacturers should aim for the best possible combination.
Conventionally, pump technology is selected first and often independently of the flow sensor, especially when unique requirements and related intellectual property of the device manufacturer are involved. Combining a previously selected pump with a liquid flow sensor can be challenging, especially when the pump performance requires further improvement, failure detection and resilience, all to be provided by the sensor. The pump’s specific working principle, flow profile, mechanical design and fluidic connectors might further complicate the task.
CONCEPT STUDY: INTEGRATION WITH THIRD-PARTY DEVICE
Sensirion has put its extensive flow sensing experience to the test in a design study, assembling a small-footprint liquid flow sensor with a single-use micropump from Quantex Arc.
Taking up only 0.5 cm3 of space (1.2 x 0.6 x 0.69 cm), Sensirion’s liquid flow sensor stands out due to its millisecond fast response times and direct as well as bidirectional flow rate measurement. The lightweight in-line pump used in the study was the Quantex Arc CS-3, which has a barbed inlet and outlet connectors for easy fitting to tubing, and is ideal for precise microdosing at flow rates of up to 100 mL/hr.
Being merely a concept study, there was surely space for further improvements and customisations, also considering different device requirements. However, the result here was a highly compact flow controller providing a steady flow with different flow regimes, and consuming very little energy.
CONCLUSION
When applied in subcutaneous LVI devices, miniaturised liquid flow sensors address the strong and accelerating market trends outlined in this article, and they do it at great value-to-cost ratio. Sensirion’s technology is compatible with pumps from numerous manufacturers, and we are at the beginning of an exciting journey.

