To Issue 155
Citation: Dugand P, Gross C, Tunkel M, “How to Navigate a Complex Connectivity Environment”. ONdrugDelivery, Issue 155 (Dec 2023), pp 6–9.
Pascal Dugand, Cécile Gross and Mark Tunkel consider the complexity of the landscape for connected devices and demonstrate how a balance can be achieved between patient needs, technology and the cost of integrating this technology into a device.
“Nemera helps to ensure that developed technologies are not only meeting market needs but that they are doing so in an optimal way.”
The methods available to pharmaceutical companies and device suppliers to support the patient journey and experience offer more possibilities than ever before. Traditionally, the focus of the patient experience was on optimising the “administration task” as outlined in the instructions for use, with an emphasis on the reduction of use error risk. However, as clinical outcomes are increasingly driving payer decision making, developers are expanding their efforts to support the patient journey more broadly.
Nemera achieves this through mapping the patient journey to develop an intimate understanding, which should be considered at the earliest stages of development. This starts from when a patient is diagnosed, to receiving their device, onboarding, acclimation, through the entire process of preparing, administering and disposal, and the times in between treatment to understand how the process changes over time and how frequency of administration may impact the patient experience.
This enables Nemera to determine how those needs can be most effectively addressed, whether through the device, the integration of connectivity, instructions or packaging materials (paper or digital), training, onboarding or other means of longitudinal engagement. Nemera helps to ensure that developed technologies are not only meeting market needs but that they are doing so in an optimal way.
This can support the trend of virtually every developer in the branded and generic space formulating some variation of a digital health approach. This often includes add-ons or accessories that are compatible with a commercially available device and provide users with device performance feedback. They can also provide error correction and other means of feedback to help eliminate use errors.
There has also been convergence within the drug delivery ecosystem, where all aspects of managing a disease state are integrated through connectivity that often requires partnerships to address broader steps in the patient journey to leverage complementary technologies (Figure 1).
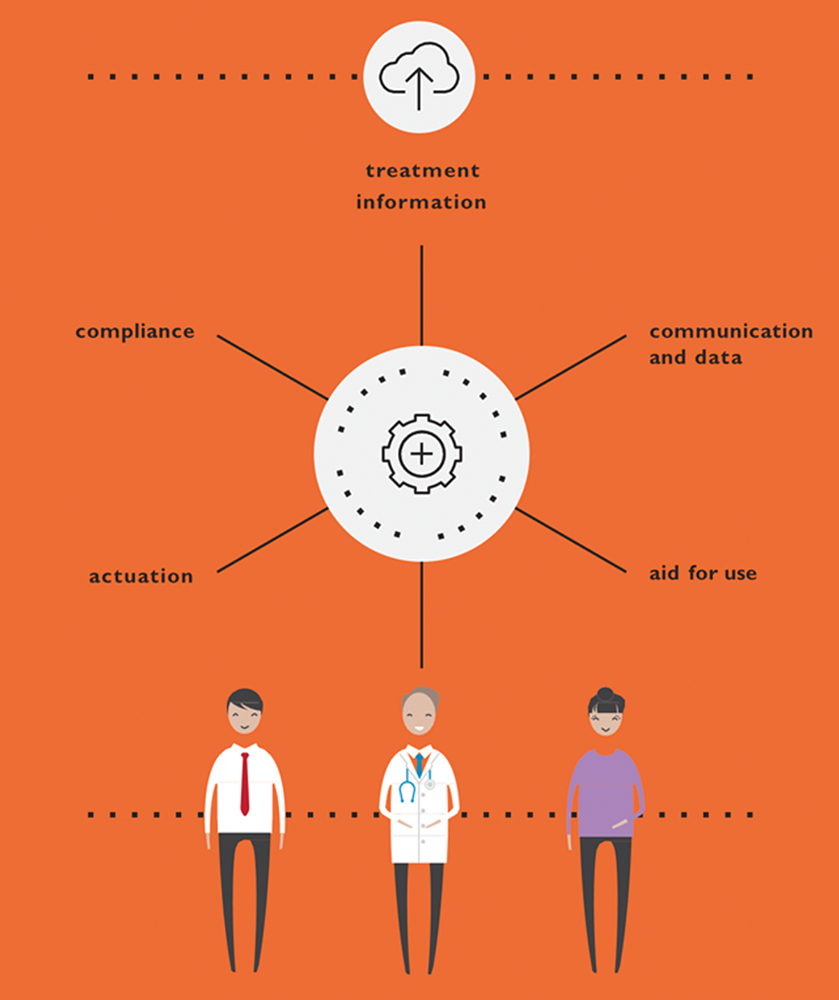
Figure 1: Connected devices.
BENEFITS OF PARTNERING WITH AN INTEGRATED PRODUCT AND SERVICE PROVIDER
Successful navigation of these challenges often requires a partner with a broad set of capabilities, products and services. Nemera’s integrated development, consulting and manufacturing services allow customers to achieve successful regulatory submission and commercial launch of safe, effective and differentiated combination products, with a single partner applying an agile process across the device and combination product value chain. Nemera supports every device strategy that a customer may consider, from organic development to the use of the company’s intellectual property (IP) platforms, and a wide range of services to support the customer’s journey towards a combination product.
Nemera’s services include frontend innovation and device development, clinical and commercial device manufacturing, and combination product services. Ultimately, the benefits of this approach will drive:
“A single partner working across the journey limits transitions between suppliers and ensures consistent execution of strategy.”
Flexibility to Focus on the Core
The value of Nemera’s integrated services lies in the flexibility it offers, allowing its customers to focus on the core business of drug discovery and development. Nemera can act as a partner and extension of customer teams and provide best-in-class solutions for devices and combination products, ensuring that no compromises are made when collaborating with the company.
Patient-Centricity and Engagement
The needs of patients and clinical stakeholders are central from the outset of the programme to develop a customised patient engagement strategy, which is consistently at the heart of the process to maximise effectiveness when launched into the market to drive stakeholder loyalty.
Innovation Across the Journey
Nemera’s world-class design, development and consulting capability ensures the deployment of innovative development strategies as unique as its customers’ applications for both the company’s IP products and custom development services, regardless of the point of engagement.
Reduction of Risk and Increased Speed of Market Access
A single partner working across the journey limits transitions between suppliers and ensures consistent execution of strategy. Nemera offers a wide range of options for developing and realising a device to accelerate the time to registration and market, spanning from small-series to large-scale manufacturing, with the flexibility to provide interim supply as needed. When combined with Nemera’s service offering, this can significantly reduce the time to market.
Nemera also offers an array of connected devices, ranging from embedded solutions with best-in-class partners operating in the field, to the customisation of an existing platform and extending an add-on solution.
SYMBIOZE™, A SMART AND SUSTAINABLE ON-BODY INJECTOR PLATFORM
Targeting mid- to long-term medication in therapeutic areas such as oncology or immunology, the Symbioze™ drug delivery system is a connected and reusable on-body injector suitable for large-volume injections. The use of such devices is more than likely to expand, as many drugs in these fields are transferred from intravenous to subcutaneous delivery, which makes the disease burden even more cumbersome for patients.
To alleviate part of this burden, the focus has been – when designing the product – on an automated and safe injection process totally invisible to the user, as well as the secure administration of the prescribed drug. Combining electronics and “proximity card” technology is Nemera’s choice to address these issues.
In the same way, a Bluetooth connectivity feature has been built in to allow functionality such as data recording and sharing, notifications and reminders. Although such a feature is common and well accepted in diabetes care, it remains innovative in cancer treatment and inflammatory pathologies.
Oncology patients need, on top of a seamless device, to achieve treatment adherence outside the hospital setting successfully, and to keep a link with remote healthcare professionals. Symbioze™ can be easily integrated into the patient journey.
To fine-tune the development of the electronics and software parts of the platform, Nemera has entered into a partnership with Zollner Elektronik (Zandt, Germany), one of the largest electronic manufacturing service providers. Zollner specialises in advanced mechatronics for healthcare and life sciences, railway technology, aerospace and defence, automotive technology and many other sectors.
As a partner of choice, it will support the design, development and manufacturing of electronic drug delivery systems for both Nemera’s proprietary and customer-owned products. This collaboration will begin with the Symbioze™ platform (Figure 2). Erwin Stöckinger, Senior Vice-President Business Division Electronics at Zollner, explained that from the start, Nemera and Zollner have been an excellent match, on both technological and human levels for the mutual development of groundbreaking solutions towards a sustainable society. The partnership will enable entry into new growth markets.

Figure 2: The Symbioze™ smart and sustainable on-body injector platform.
In the same way, Nemera is expanding its view of the patient journey beyond the treatment journey to embrace the patient’s perspective more holistically; adding sustainability is a means for Nemera to show its commitment as a company to the climate, as well as being consistent with its motto, “We put patients first”.
Figure 3: Customised Safe’n’Sound® with NFC tag.
LOOKING INTO THE FUTURE: CUSTOMISED AND ADD-ON CONNECTIVITY
Keeping in mind patient adherence and looking for an innovative means to alleviate the burden of regular self-injections, it seemed natural to Nemera’s technical teams to review the customisation possibilities on its safety system platform Safe’n’Sound®. The selected solution was to incorporate a near-field communication (NFC) connectivity tag into the device mechanism. Given its extremely large capabilities of data retrieval and transmission, the first prototype has been built upon data related to injection completion and injection history. The NFC tag has been customised to allow two device configurations: before and after injection (Figure 3).
The initial phase prior to injection aims to reassure patients that the device has never been used and to check the authenticity and expiration date of the drug. Once the device has been activated and the injection performed, this information is integrated into the patient’s injection history, confirming a full injected dose. No specific application has to be downloaded to store the data as, by simply placing the device near the phone, all data are easily accessible through a web browser.
With high traceability features, this technology holds significant value, including for clinical trials. On the topic of clinical trials, an integrated connected pen is also available. Customised from Nemera’s reusable pen platform Pendura AD, the device features a Bluetooth module integrated into the pen itself, which can help transfer data to an app. Before transferring it, the data related to the injected dose are displayed onthe screen, together with the date and time of injection. Complete data are available in the history on the application (Figure 4).
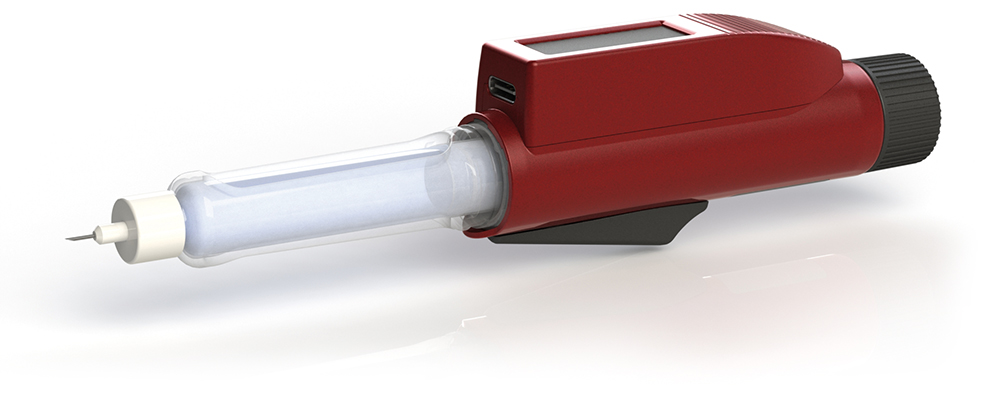
Figure 4: Integrated reusable connected pen injector, ideal for tracking injected doses during clinical trials.
“To avoid underdosing or overdosing, Nemera’s pen injectors can be customised with a specialised add-on.”
Another area of interest is to record the number of doses injected. When the treatment becomes regular, sometimes even daily, patients have difficulty following up their regimen. Within the ageing population, many individuals often undergo multiple treatments, making it challenging for them to remember whether they have taken their prescribed doses. To avoid underdosing or overdosing, Nemera’s pen injectors can be customised with a specialised add-on. Designed to assist patients in monitoring their injections, it collects details of injected doses and adds them into the history. The main point is to accurately count the number of administered doses by recognising key parameters such as typical pen use sequences, pen position and the distinctive injection dose signature while detecting each specific step of priming and injection (Figure 5).

Figure 5: Specialised add-on for disposable pen injector, enabling key parameters detection to count administered doses accurately.
Finding the right balance between patient needs, technology and the cost of integrating this technology into a device as smoothly as possible is no easy task. Nemera strongly believes in its experience, its internal capabilities and its renowned partners to offer tailored solutions to patients worldwide.
Symbioze™ is a registered trademark of Nemera La Verpillière SAS in the EU. All picture copyrights belong to Insight by Nemera

