To Issue 136
Citation: McDermott B, Krusniak S, “Industrialisation and Scale-Up of Drug Delivery Devices”. ONdrugDelivery, Issue 136 (Aug 2022), pp 6–9.
Barry McDermott and Shari Krusniak look at the manufacturing processes involved in bringing a new drug delivery system and formulation to market.
Over the last five years, revenue coming from biologics and biosimilars has increased significantly. This rapid growth has inspired many pharmaceutical companies to invest in biotherapeutics. However, these drugs are sensitive and complex and require expertise in their development, containment and delivery systems. In fact, these drugs commonly start in a standard format but evolve into more convenient prefilled syringes or combination product wearable devices with the goal of improving market share, product differentiation, and patient experience and adherence. For these complex devices, meeting these goals requires great expertise and reliance on a strong supply network.
Drug delivery devices often include technology that must meet regulatory standards and remain current in the fast-paced and evolving market. When considering a novel drug delivery system combined with a new formulation – with either one or both requiring the development of manufacturing processes – considerable challenges are involved in meeting the required quality standards and scaling up the required resources while containing costs and minimising time to market.
Typically, pharmaceutical companies focus on their expertise in the development of the therapy itself and then collaborate with manufacturing partners to research, design and develop the containment and delivery system of the drug to meet those requirements. A successful partnership between these companies is essential for the manufacturing of a safe, effective and competitive therapy to accelerate market entry and to respond quickly to the constantly changing landscape (Figure 1).
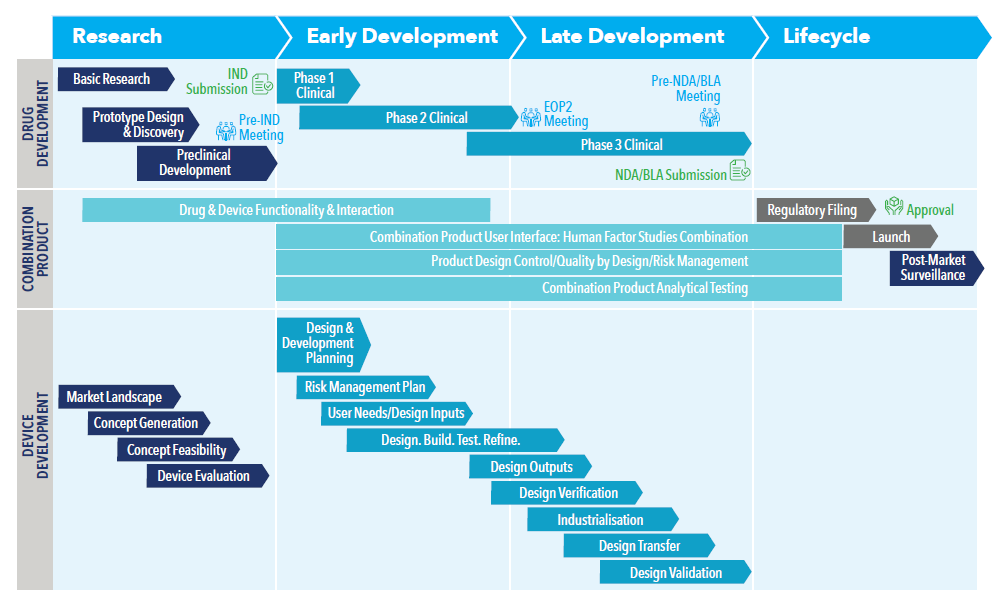
Figure 1: Combination product development process. Bringing a biologic to the market requires that the drug development process and the device development process come together as early as possible. Considering drug and device separately can introduce significant risk.
“Moving through the development process, West optimises the key critical attributes for quality to confirm a robust design and capable processes.”
COLLABORATION IS CRITICAL
To reach the goals of your development programme, it is important to partner with a trusted device supplier or contract manufacturing organisation. From concept to design, the partnership needs to include early and frequent collaboration and communication, protection of intellectual property and consideration of the patient’s full journey. Both partners also need to be aware of challenges that can arise and address root issues through close collaboration.
Many challenges can hinder progress towards delivering innovative, patient-centric products, but those that arise when there is conflict between a pharmaceutical company and its manufacturing provider are often overlooked until they begin to slow the process. These issues can arise in any partnership and can be compounded when teams come from different companies and work cultures. Misaligned goals, processes and expectations need to be addressed early to establish a successful partnership. Lack of accountability and decision making, as well as poor communication and problem solving, are common issues that may lead to missed deadlines and/or poor-quality products. With patient safety on the line, it is essential to collaborate successfully and communicate regularly to address problems when they arise.
YOUR DRUG AND ITS DELIVERY DEVICE: A CO-ORDINATED DESIGN, DEVELOPMENT AND MANUFACTURING PERSPECTIVE
Biopharmaceutical companies tend to focus on the molecule of interest and its functional characteristics, but it is equally important to consider how ultimately that molecule will be manufactured from lab to commercial scale, taking into consideration its containment system and how it will be delivered to the patient. As a manufacturing partner, West offers solutions for the design of drug delivery devices that optimise manufacturability. The company understands the importance of transitioning a device from development to the manufacturing stage, which must be done with an understanding of the regulatory needs, scale and expansion of the product in the best interest of the customer. These priorities and West’s decades of experience have made the company a trusted solutions provider for many successful pharmaceutical companies. West’s approach to manufacturing involves several key aspects:
- Strong programme management
- High standards of quality through design for reliability and capability (DRC)
- Knowledge of changing regulations
- Analytical testing and data to satisfy regulatory agencies
- Capacity for product scalability
- Proper drug handling and cold chain solutions.
Strong Programme Management
Programme management refers to the ability to manage risks while providing timely solutions to immediate challenges and anticipating future needs. A programme kick-off meeting will clarify the needs of both partners and the drug delivery device, and a strong management practice will help to incorporate those needs into the project plan. The programme manager oversees all phases of programmes, from inception through completion, ensuring projects are completed on time and within budget. This person is responsible for scheduling and technical performance and performs the following duties:
- Co-ordinates cross-functional project/programme teams from design to delivery of fully developed healthcare products ready for customer use
- Provides technical support in executing the project plan to meet customer requirements
- Monitors performance and recommends schedule changes, cost adjustments or resource additions
- Co-ordinates the design, build and validation of tooling, assembly line and supply chain.
“West designs modern devices and, with early and frequent collaboration with the pharmaceutical company, can adapt them to meet changing regulations that may arise during the manufacturing process.”
High Standards of Quality Through DRC
As a global contract manufacturer in the development of devices supporting the pharmaceutical industry, West works with customers to ensure its devices are designed for reliability and capability. Reliability refers to product reliability and robustness, while capability refers to the ability of a product to be assembled and manufactured and to function for its indicated use.
As DRC starts at the research/concept phase of the product development process, West incorporates quality and robustness from the very beginning and, ultimately, optimises the product design and associated manufacturing process for scale-up. West follows its defined DRC methodology, starting with understanding the voice of the customer and translating that voice to concept and component generation and selection while incorporating its design for manufacture and assembly expertise for internal and external supply.
Moving through the development process, West optimises the key critical attributes for quality to confirm a robust design and capable processes. As manufacturing plans are established and product design validation testing and process qualification is completed, the company prepares for production and commercial supply. This established methodology manages the risks of time and cost creep by anticipating and managing potential issues, as well as building quality into the design of the customer’s new product. Thus, moving from problem solving to problem prevention.
The process collects vast amounts of data to reveal even subtle changes that affect product integrity, and this approach is consistent across both the product and process development. Data collected as part of the product development and manufacturing process are integral to optimising product and process design and in developing leading, rather than lagging, metrics that guide product and project management, training, infrastructure and capital needs. The DRC methodology, which incorporates data collection and analysis, can provide critical insights that:
- Identify defects earlier
- Reduce manufacturing scrap
- Reduce product variation
- Improve outgoing quality and on-time delivery
- Increase customer satisfaction.
Knowledge of Changing Regulations
One challenge to anticipate is the ever-changing regulatory landscape for drugs and their delivery systems. West stays up to date with these changes and aims to manufacture devices that exceed current standards in both the physical and technological design of the system. As wearable devices and self-administered products employ the most current technology, West is constantly researching the regulations, including those outside the drug industry, that your product and its electronics must meet to reach the market quickly and safely. West designs modern devices and, with early and frequent collaboration with the pharmaceutical company, can adapt them to meet changing regulations that may arise during the manufacturing process. By leading with the company’s quality-by-design process, this requirement is met even through design adaptations to provide safe and effective drugs.
CASE STUDY: NOVEL DRUG DELIVERY DEVICE
A pharmaceutical company and their product design partner chose West as their manufacturing solutions provider for a breath-actuated inhaler with an incorporated dose-counter mechanism and a metered canister of pressurised drug suspension to treat patients with asthma and chronic obstructive pulmonary disease. Thinking ahead, the development team designed and manufactured the device to become a platform technology for future therapies.
West provided support in device development, injection moulding, tooling, supply chain logistics and the scale-up automation strategy. This support resulted in improved risk mitigation, speed to market and a simplified supply chain during the process of manufacturing these devices at clinical trial scale, with the capacity to scale up to meet the needs of the commercial market. However, the success of the partnership and final combination drug manufacturing did not come without challenges and the need to adapt to changing requirements.
Challenges included scaling up from single-cavity tools to multi-cavity tools, adhering to tight tolerances, moulding and assembling multiple components with new materials while meeting customer specifications, meeting critical processing parameters, developing an integrated automation solution for the device assembly and verifying the device’s functionality. These challenges were addressed through West’s comprehensive plan that included conceptualisation, design, mould manufacturing and adherence to regulations.
The final respiratory device consisted of 12 injection-moulded components produced from four different resin groups through a process that involved building seven components into a dose-counter assembly. Engineering verification testing then involved design alterations to ensure the robustness of the device during typical patient use.
The result achieved by West was the delivery of 11 of the 12 multi-cavity tools to progress to the quality control stage. This involved design of experiments, metrology, steel cuts if required, process capability and corner process studies, and metrology development and review. The development also included automation of the assembly to ensure scalability, all while adhering to the highest standards of compliance and quality.
Analytical Testing and Data to Satisfy Regulatory Agencies
Access to robust analytical testing services is critical for successful commercialisation of biologics. Most pharmaceutical and biotech companies outsource these services because of the need for highly specialised staff and equipment to perform injectable package testing to satisfy regulatory demand. West has extensive experience in extractables and leachables, particle analysis, container closure integrity, and performance and packaging/delivery systems, among other methodologies. The company’s dedicated packaging and performance testing group works with customers to develop custom protocols specific to their needs – and the needs of the combination drug product. West partners with clients and understands the current regulations and standards as well as ensuring the proper study design and interpretation of the results.
Capacity for Product Scalability
With a product designed and manufactured, scalability must also be considered. It is important to understand the needs of the market for your specific drug and if there are potential hurdles in the supply chain that may slow the process of scaling its production. As an experienced solutions provider, West knows how to anticipate hurdles and designs products, including the required tooling, equipment, facilities and clean room capacity, from the start to minimise such risks.
Proper Drug Handling and Cold Chain Solutions
In addition to the supply chain for the device itself, you and your manufacturing partner must consider the requirements for the drug’s delivery and storage. Biotherapeutics are sensitive drugs typically containing unstable molecules that require temperature-controlled handling, storage, transportation and dispensing. It is crucial that your contract manufacturing provider can ensure proper cold chain solutions so as to not compromise the integrity, efficacy, safety or security of the drug.
WEST IS A TRUSTED MANUFACTURING PARTNER
Today, biotherapeutic drug delivery is trending towards self-administration and improved patient experience, requiring sophisticated drug delivery devices, such as self-administered injectables and other types of combination products. The industrialisation needs of modern delivery systems are more complex and require an experienced manufacturing partner and a proven track record of helping pharmaceutical companies bring medicines and products to the market.
With more than 100 years of experience providing packaging, transportation, storage and manufacturing of drug delivery devices, West has collaborated with pharmaceutical and biotech companies around the world to create market differentiating drug delivery systems for their products. The success of these partnerships has resulted in the use of more than 112 million West components and/or devices each day, with eight out of 10 of the biggest-selling biologics relying on West packaging.

