To Issue 179
Citation: “Interview with Fabien de Coninck and Bruno Morchain, Aptar Pharma: a Structured Approach to the Eco-Design Process”. ONdrugDelivery, Issue 179 (Oct/Nov 2025), pp 28–31.
Q What is Aptar Pharma’s strategy when it comes to sustainability?
BM As a part of AptarGroup, Aptar Pharma places sustainability at the core of its business strategy, integrating environmental, social and economic considerations into its policies and operations. The aim of this approach is to create long-term benefits for the company and its employees, while being mindful of conserving and protecting resources.
Aptar’s corporate sustainability strategy is built around three key pillars – care, collaboration and circularity. Based on this strategy, we focus on employees, communities and the environment by continuously improving our impact and reducing our carbon footprint. Furthermore, we seek to design our products with people and the planet in mind, while also incorporating eco-design principles to contribute to a more circular economy. This means understanding the key lifecycle impacts of our products and innovating to deliver optimal performance throughout the value chain.
Aptar Pharma has embraced this strategy as evidenced by the continued development of new products within our Futurity™ sustainable solutions platform. The Futurity™ platform meets the growing need for recyclable packaging and drug delivery solutions in the pharmaceutical industry, supporting our customers as they improve circularity for their products.
Q How does Aptar Pharma’s injectables segment align with the wider company’s global sustainability strategy?
BM Our injectables primary packaging components – stoppers for vials; and plungers and tip closures, including rigid needle shields, for prefilled syringes – are made from rubber (Figure 1). Working with this type of material requires a specific process, notably including the compounding of several raw materials, such as synthetic elastomers, mineral fillers, curing agents and pigments, to form a hardened cross-linked polymer. This makes it difficult to recycle in existing recycling streams.

Figure 1: Aptar Pharma’s moulded rubber stoppers.
In addition to this, primary packaging components are often in direct contact with the drug and must be used in conjunction with glass containers. As glass containers and elastomeric closure components come into direct contact with patients – whether via the intravenous, intramuscular or subcutaneous route – packaging solutions for injectable drugs are considered biohazardous waste and treated as such.
Under the current pharmaceutical waste management paradigm, rubber components are unlikely to enter any recycling stream after being used. Therefore, we have had to find other levers to fulfil our sustainability objectives. As there are challenges with changing the design or implementing recycling for these specific types of products, we have turned to looking at our corporate environmental footprint to make tangible progress. Within the company, we’ve implemented dedicated initiatives to reduce the environmental footprint of our sites, which can, in turn, help our customers reduce their Scope 3 emissions.
All of Aptar Pharma’s injectables manufacturing sites are certified as landfill free by our internal certification programme. To obtain and maintain the certification, sites are required to prove that they reuse or recycle at least 90% of their operational waste through a third-party audit. Aptar’s landfill-free programme contributes to environmental sustainability by aiming for continuous improvement in waste reduction. Within Aptar Pharma sites, we have worked to establish a dedicated recycling network for vulcanised rubber waste, enabling us to recycle at least 90% of our rubber waste annually.
“AS PART OF OUR GLOBAL EXPANSION, WE HAVE BEEN IMPLEMENTING ENERGY-SAVING INITIATIVES, AIMING TO REDUCE NATURAL GAS CONSUMPTION AT OUR FACILITIES WORLDWIDE.”
Additionally, all of the electricity we use within our injectables sites comes from renewable sources. Most importantly, as part of our global expansion, we have been implementing energy-saving initiatives, aiming to reduce natural gas consumption at our facilities worldwide. For example, by implementing a state-of-the-art steam boiler in our Granville (France) factory, modernising utilities and implementing heat-recovery mechanisms, we’ve reduced the consumption of energy for building heating and production.
Turning to our product development strategy, we have proactively launched an initiative to integrate eco-design into our development process, anticipating future regulatory changes, such as perfluoroalkyl substance restrictions and future packaging regulations. The first milestone in this approach is conducting a lifecycle assessment (LCA) of our products.
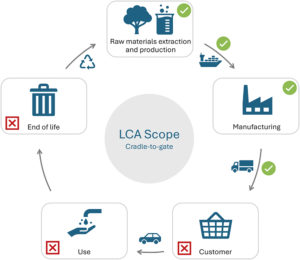
Figure 2: Scope of a cradle-to-gate LCA.
Q What does a lifecycle assessment consist of?
FDC Conducting an LCA of existing products is a key step in developing a robust eco-design strategy. LCAs provide a comprehensive evaluation of a product’s environmental impacts across its entire lifecycle – from raw material extraction to end-of-life disposal (Figure 2). By identifying the most impactful stages and processes, we can then prioritise areas for improvement.
This initiative is part of Aptar Group’s company-wide strategy to conduct LCAs systematically for every new development project. In our case, we have implemented an advanced cradle-to-gate LCA, covering all stages from raw material production to delivery to the customers for each product range. This approach enables us to accurately identify environmental “hotspots” and guide our eco-design actions more effectively.
“THE RAW MATERIALS USED IN OUR RUBBER FORMULATIONS ARE THE MAIN CONTRIBUTORS TO OUR CARBON FOOTPRINT, ACCOUNTING FOR UP TO 75% OF THE OVERALL ENVIRONMENTAL IMPACT OF OUR COMPONENTS.”
In the field of rubber components for injectable devices, this is particularly innovative – our rubber formulations are proprietary, and our manufacturing processes are complex. As a result, the generic datasets present within existing LCA software and methodologies are not representative of our products. Therefore, we have adapted our LCA tools to consider the specificities of each rubber formulation we use and the steps of our manufacturing process.
Q How did you ensure that the LCA of your products was implemented effectively?
FDC Aligned with ISO 14040 and 14044 guidelines for LCAs, we conducted our study using the well-known Sphera® LCA FE software, previously named “GaBi” (Sphera, Chicago, IL, US). We focused on a cradle-to-gate scope, which includes the product lifecycle steps from raw materials production to final delivery to the customer, so it covers our production steps, the secondary packaging materials and supply transport. Our approach was to build a flexible and customisable LCA model, considering various scenarios and parameters to enable it to match the lifecycles of our various injectables products. An automatic report with standard environmental results was linked to the model to facilitate fluent analysis. Following our own initial analysis, we are now preparing for an LCA review by a third party in early 2026.
Q What were the main challenges you encountered during the LCA process?
FDC Throughout this process, data inventory was a challenging step. It required a significant time investment to ensure our analyses were based on truly reliable data. The development of our LCA tool was a six-month full-time project led by an eco-design engineer and supported by a multidisciplinary team, primarily from the R&D, environment, health and safety, maintenance and procurement departments.
Initially, the lack of supplier data and the challenges in accessing it proved to be a barrier to our approach. Nevertheless, we observed a genuine awareness among all stakeholders in our ecosystem about the importance of assessing their environmental impact. Thanks to specific confidentiality agreements, we were able to initiate constructive exchanges to share environmental impact data and align data transfer practices that supported a robust and detailed LCA.
Q What are the key conclusions from the LCA of your products?
BM Based on our preliminary results (Figure 3), the conclusions are clear – as we had expected. The raw materials used in our rubber formulations are the main contributors to our carbon footprint, accounting for up to 75% of the overall environmental impact of our components. Among these, elastomers alone represented an average of 85% of the rubber compounding impact. Overall, we observed a carbon footprint ranging from 4–7 kg carbon dioxide equivalents (CO2eq) per kilogram of rubber components.
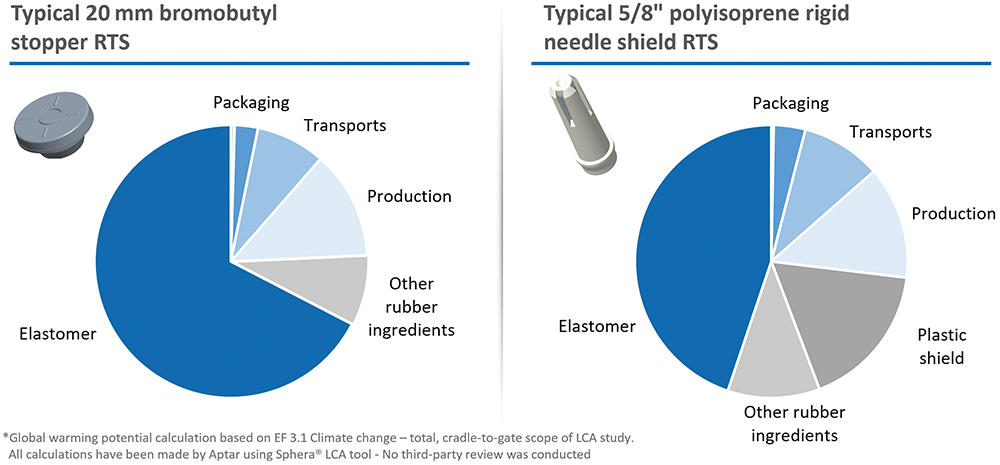
Figure 3: Global warming potential of elastomeric closures (RTU: Ready-To-Sterilise).
As an example, let’s consider a 13 mm stopper for a standard 2R borosilicate glass vial to compare the carbon dioxide emissions within our defined cradle-to-gate scope. The global warming potential for this rubber stopper is evaluated to be around 3 g CO2eq per unit and the glass vial around 28 g CO2eq per unit, based on the preliminary LCA and estimations. Data on the glass component was obtained from Corning (Corning, NY, US) based on their optimised Viridian™ vials.
The impact of our manufacturing process, primarily energy and resource consumption, on the carbon footprint remains moderate, thanks to the various measures implemented at our production sites in recent years, such as the use of renewable energy and water-saving initiatives. Other impact categories – including transport, packaging and non-product-contact materials such as the polypropylene shell of rigid needle shields – have a limited footprint but still present interesting short-term opportunities for improvement. This insight is now driving our material innovation efforts towards lower-impact alternatives, without compromising the stringent regulatory and functional requirements of parenteral applications.
“OUR LCA TOOL NOW ENABLES US TO QUICKLY ASSESS THE ENVIRONMENTAL IMPACT OF VARIOUS PRODUCT DEVELOPMENT SCENARIOS WITHIN OUR INNOVATION PROJECTS.”
Q How does the LCA help you define or validate your eco-design strategy?
FDC We keep a strong commitment to eco-design at the heart of our materials strategy. Every new development is evaluated for environmental impact – reducing material usage, lightweighting, enhancing durability and replacing high-impact components. Our LCA tool now enables us to quickly assess the environmental impact of various product development scenarios within our innovation projects.
Putting this into practice, we primarily focus on the hotspots identified through the LCAs, combining both short and long-term initiatives. The LCA results highlight the need to address raw materials as the primary contributor to carbon footprints. This is a critical area where development and implementation timelines with pharmaceutical partners tend to be long, as it may affect their product validation and stability studies.
“WE ARE PARTICIPATING IN A MULTI-PARTNER RESEARCH PROJECT ON BIO-BASED MATERIALS IN THE RUBBER SECTOR, CALLED BIOPROOF 2, LED BY ELANOVA.”
Our R&D sustainability roadmap is structured around four key pillars: raw materials, waste management, process improvement and secondary packaging. In terms of materials, we’re currently exploring initiatives related to the use of renewable mass balance certified materials. We’re aiming to reduce the use of fossil-based materials and have launched research into alternative materials to make this reduction easier. Notably, we are participating in a multi-partner research project on bio-based materials in the rubber sector, called Bioproof 2, led by Elanova (Vitry-sur-Seine, France).
We have other initiatives underway to reduce material usage by optimising component design, all while maintaining critical functions such as tightness performance, container closure integrity and functional properties during piercing.
Q How does this approach support Aptar’s customers?
BM Firstly, calculating the environmental impact of a product is becoming essential. In some industries, this process is mandatory and well-regulated. In the pharmaceutical sector, there isn’t a legal obligation yet, but requests from our customers are becoming increasingly frequent. Aptar’s emissions are accounted for as Scope 3 emissions in our customers’ greenhouse gas inventories, so we need to understand the full value chain impact to take action where it can be the most impactful.
Current customer requests are often focused on calculating a product’s global warming potential and understanding where progress can be made in reducing these potential impacts. We believe that restricting LCAs to emissions alone for rubber components does not provide a sufficiently accurate picture, due to the unique characteristics of the material and the specifics of the production process. Therefore, we consider additional environmental impact categories, such as primary energy demand and fossil resource depletion, to provide a more comprehensive assessment.
Additionally, some clients now request detailed environmental impact categories based on specific methodologies, such as the European Environmental Footprint. It is therefore crucial to be prepared to meet these specific needs and to stay up to date with evolving industry trends. As a supplier, we play a vital role in creating a complete and accurate LCA of the final product that covers all stages of its lifecycle.
Secondly, understanding the LCA results of our products, coupled with our own internal corporate sustainability strategy, allows us to quickly initiate actions that contribute to reducing the environmental impact of the final product. It empowers us to take a proactive approach to manufacturing more responsibly and prioritising our eco-design strategies in alignment with a shared goal of our clients, reducing the negative environmental impact of products.
Q What are the upcoming steps in your process?
BM We plan to include a critical review phase of our LCAs by a third party before sharing data externally. Additionally, we aim to leverage the full potential of LCAs as a decision-making tool to help prioritise our eco-design projects. This will also enable us to quantify and highlight the improvements made to our manufacturing processes more accurately.

