To Issue 158
Citation: “Interview with Julie Suman, Aptar Pharma: Exploring Nasal Casts in Nasal Drug Development”. ONdrugDelivery, Issue 158 (Apr 2024), pp 78–81.
“The introduction of 3D printing has made it possible to manufacture nasal casts from images of living patients or volunteers, which is the approach that is now both favoured and increasingly accessible.”
Q Can you provide an overview of the historical development and evolution of nasal casts? What were the primary motivations and objectives behind their creation?
A The first nasal casts were modelled directly from cadavers – a logical strategy. The most well-known of these is probably the Koken Co silicone cast, which is still commercially available. It should be noted that casts made from cadavers have a more patent nasal cavity and are not the best representation for studies. Nonetheless, over the years, many such casts have been referenced in the literature, typically developed in-house by individual research groups or companies.
The introduction of 3D printing has made it possible to manufacture nasal casts from images of living patients or volunteers, which is the approach that is now both favoured and increasingly accessible. Computer tomography (CT) or magnetic resonance imaging (MRI) images gathered from the patient population of interest are typically the starting point; beyond which, techniques such as stereolithography enable realisation of a nasal cast with ever greater precision, from a plastic or polymer of choice.
Nasal cast use dates back to the 1990s, and the goal from the outset was simply to gain a better understanding of where in the nose a drug might deposit and how that deposition might vary between and across different patient cohorts. Teams such as that led by Dr Richard Dalby, Professor in the Department of Pharmaceutical Sciences at the University of Maryland School of Pharmacy (MD US), pioneered the use of nasal casts to visualise spray deposition for educative purposes, as well as for product development. The use of coloured sprays and tests where casts have been coated with products such as SarGel®, a white paste that turns purple when in contact with water, produce effects that are both visually arresting and informative; nasal casts have delivered some excellent images for marketing purposes over the years!
Q How are nasal casts used in nasal deposition studies, and what advantages do they offer over alternative methods for studying nasal deposition?
A Current regulatory guidance for nasal drug products specifies in vitro techniques that usefully quantify important metrics, such as particle size, spray pattern and plume geometry. These metrics relate to nasal deposition but do not measure it directly.
Direct measurement is possible via in vivo testing with techniques such as a gamma scintigraphy, or using a CT or positron emission tomography (PET) scanner to track the distribution of a radioactive tracer. However, such testing is relatively expensive, time-consuming and inaccessible.
Nasal casts essentially bridge the gap between these two approaches. Using them enables the direct study of nasal deposition in the lab, with no requirement to recruit volunteers (and obtain the necessary consent) or have access to CT and PET scanners, which are in near constant clinical use at many hospitals (Figure 1).
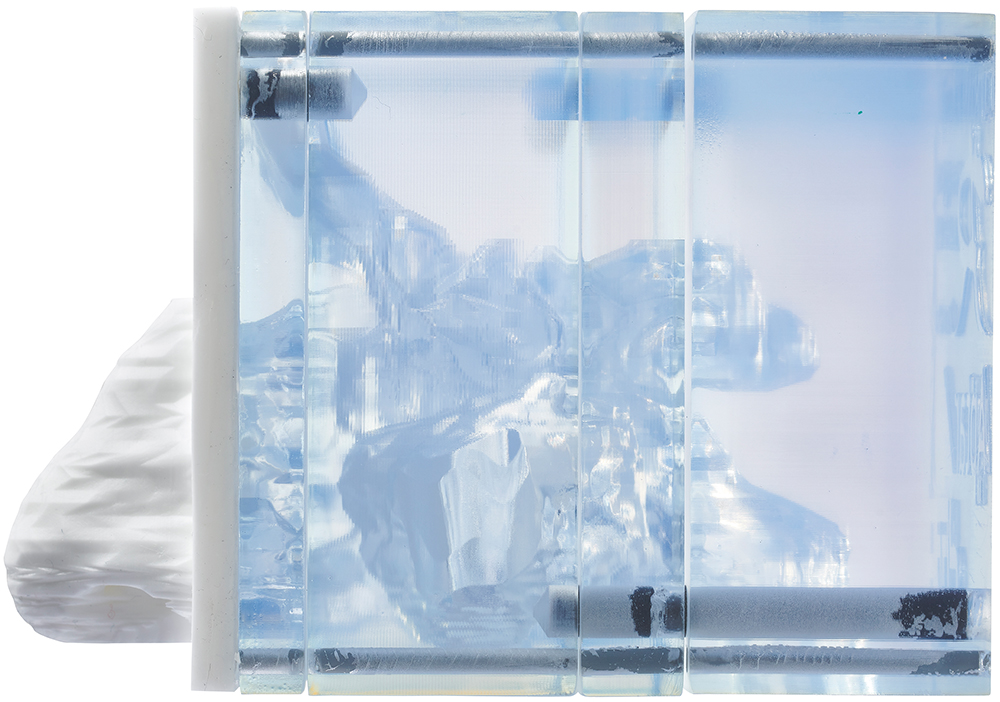
Figure 1: Schematic view of Aptar Pharma’s Aeronose nasal cast modelling.
Q How do you select a nasal cast? And what about validation?
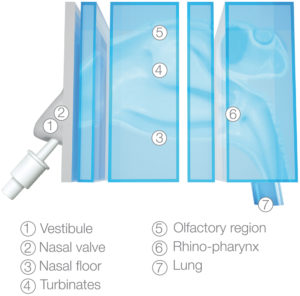
Figure 2: Aptar’s Aeronose nasal cast for liquid sprays and nasal powders.
A It is important to select a nasal cast that is representative of the patient population that you are targeting – paediatric, adult or geriatric. The physiology of the nasal cavity varies considerably with factors such as age, gender, ethnicity and disease state, so having a cast that is as representative as possible is important. Currently, there is no standardisation for nasal casts, so careful selection and validation are critical.
The other thing to consider is that nasal casts are often sectioned, so make sure you can access the area of the nasal cavity that you are specifically interested in. This might be more difficult if you’re targeting a small region, such as the olfactory region, as is often the case for those working on nose-to-brain drug delivery.
Validation against in vivo data is essential to ensure the relevance of your model and give it weight. Using models that are already validated, via academic collaboration or service providers, can therefore prove both cost and time effective. For example, Aptar Pharma offers access to its Aeronose™ nasal cast, which was developed by Aptar Pharma in collaboration with the University of Tours (France) and has already been validated with both liquid sprays and nasal powders (Figure 2).
Q Could you elaborate on the role of nasal casts in predicting in vivo deposition patterns? What insights do they offer that are crucial for understanding how particles or drugs interact with the nasal cavity?
A Looking across different areas of nasal drug product development activities can help to elucidate the need and scope for application for nasal casts; our collective motivation for developing a better understanding of in vivo deposition behaviour is growing. For example, for those working on generics, nasal cast studies inform the closer matching of drug delivery performance and can add further weight to claims of bioequivalence, as exemplified by the contested approval of Sandoz’s (Basel, Switzerland) Mometasone Furoate Sandoz (mometasone furoate) nasal spray, which was supported in the EU by nasal cast data. Currently, the US FDA does not use nasal cast data to make regulatory decisions; it is considered part of the development package.
“For those working on generics, nasal cast studies inform the closer matching of drug delivery performance and can add further weight to claims of bioequivalence.”
For systemics, on the other hand, targeted deposition may be essential for therapeutic efficacy. We are some way from robustly understanding where – or indeed how – to target nasal drug delivery to optimise clinical efficacy for different therapies; we still need better nasal deposition data to help us to build the knowledge base. Currently, nasal casts don’t address mucociliary clearance, which could impact absorption.
However, nasal casts are good first step and potentially a cost- and time-efficient way of obtaining these data.
Focusing specifically on vaccines, these are notably of interest for paediatrics, so we need to understand how deposition is influenced by physiology as it changes throughout a child’s early years. Last, but not least, realistic deposition data is essential for the estimation of off-target delivery to the lungs and the associated safety risks.
Q What challenges and limitations impact the faithful reproduction of nasal anatomy?
A A well-recognised problem with nasal casts developed from cadavers is that blood loss and tissue retraction substantially alter the geometry of the nasal cavity. Such casts are routinely reported as having overly wide nasal passageways and large nasal volumes, relative to live subjects, and can consequently produce results that are poorly representative of in vivo behaviour.
While, with modern techniques, more representative casts can be produced, there are still limitations. It is not straightforward to translate a CT or MRI scan into a 3D print, though know-how is improving, and with a high-performance CT/MRI scanner and 3D printer, the nasal anatomy can now be reproduced in fine detail. That said, material choice can be tricky, with most casts significantly removed from the flexibility and surface texture of human anatomies. Truly faithful re-creation of the nasal cavity is always going to be complex, given its highly convoluted geometry and many nooks and crannies.

Figure 3: Side view of the Aeronose nasal cast being used to study deposition in the nasal cavity.
Q In what practical applications are nasal casts most useful?
A Nasal casts are a great R&D tool for tasks such as ranking formulations or refining device design. They can help you to better decide which candidate formulation is most likely to be successful and to understand how to improve a device to target a specific deposition profile or achieve parity between a reference and test product. Such tests have been helpful, for example, for exploring the regional deposition disparities associated with liquid and dry powder nasal delivery, adding to the weight of evidence that powders offer enhanced delivery to the olfactory region (Figure 3).
Instructions for nasal product use tend to be relatively vague but, with nasal casts, we can investigate the effect of patient-use parameters, such as spray orientation, insertion depth and inspiratory flow rate. We can use these results to offer more definitive advice on product use and assess the benefits of design features such as nasal guides. We can also assess performance for different patient groups, including paediatrics of different ages, provided we have appropriate casts.
The bottom line is that nasal cast experiments are quick and relatively easy. With a validated cast, stepping through a battery of alternative options to determine the best path forwards is straightforward and inexpensive and can really help to accelerate progress.
“The primary value of nasal casts lies in their utility for rapid screening, for education and for device and formulation selection.”
Q Can you summarise the key strengths and weaknesses associated with using nasal casts as research tools? Are there any ongoing innovations or developments that will address them?
A The primary value of nasal casts lies in their utility for rapid screening, for education and for device and formulation selection. We know that nasal casts can provide a good prediction of in vivo behaviour, and that they can therefore provide clinically relevant information for the population of physiologically based pharmacokinetic (PBPK) models. The ability to visualise spray deposition is also extremely valuable.
With respect to limitations, individual nasal casts are unable to capture intersubject differences in anatomy that may be relevant to therapeutic efficacy. They are also static devices with no ability to mimic the dynamic behaviour of the nose with respect to the changes in geometry associated with inhalation and exhalation and, critically, the effect of mucociliary clearance. The other key drawback is that, in the absence of standardisation, comparing results obtained with different nasal casts or across multiple labs is not feasible.
Regulatory requirement would be a game changer for nasal cast use and would likely help to address the issue of standardisation. FDA grants to Virginia Commonwealth University to develop adult and paediatric nasal models to capture nasal deposition profiles signals an awareness of this issue, and views from a US Pharmacopoeia expert panel have now been published as well. These highlight the potential to use nasal casts for the direct assessment of in vivo nasal deposition and to more realistically assess the risk of off-target delivery to the lung, concluding with a recommendation to examine nasal casts for regulatory use.
Standard nasal casts for specific patient populations made using prescribed methods would allow inter-study, inter-lab data comparison and support more rapid development of nasal drug delivery knowledge. Such developments, in combination with steadily improving in silico skills, notably with respect to PBPK modelling, could reduce our reliance on animal testing – a major gain. The development of representative non-human primate nasal casts would be an important step in this regard (Figure 4).

Figure 4: Positioning the Aeronose nasal cast.
There are also moves to further enhance the clinical relevance of nasal casts. The use of artificial mucus and coatings, humidification and flexible nose pieces are noteworthy, and we’ve also seen researchers combine in vitro RPMI 2650 nasal cell culture models into 3D models to study permeation alongside deposition.
Q Any last thoughts?
A I’ll be really interested to see where we’re at with nasal casts in 5 to 10 years. Growing interest in intranasal drug delivery, the potential for regulatory change and rapidly advancing PBPK modelling skills could combine to substantially impact how we carry out in vitro testing and its value. I’m sure nasal casts will become increasingly routine as we work out how to minimise the cost and time associated with developing and commercialising a steadily diversifying array of nasal drug products.
To find out more about Aptar Pharma’s work in nasal drug delivery, visit: aptar.com/pharmaceutical/delivery-routes/nasal-system-drug-delivery-oindp.

