To Issue 138
Citation: Buchine B, “It’s Time to Level Up Lyo – Introducing a Swift Shift in Dual-Chamber Autoinjector Technology”. ONdrugDelivery, Issue 138 (Oct 2022), pp 65–68.
Brent Buchine discusses the need for automated, easy-to-use reconstitution devices, especially in lyophilised medications, and introduces Windgap Medical’s technology platforms, which rise to the challenge.
“More than half of injectable drugs will soon require lyophilisation, creating new challenges and opportunities for drug delivery.”
In the pharmaceutical industry, the rapid rise of injectables has been impossible to ignore. These drug products offer an alternative to oral medications, and are changing the landscape of the pharmaceutical industry. The growing prevalence of chronic diseases, a focus on developing cost-effective treatments and drug shortages continue to drive market growth.1 According to Precedence Research, the global injectable drug delivery market reached US$561 billion (£483 billion) in 2021 and is expected to surpass $1,224 billion by 2030.
Increasingly prevalent in the injectables space are lyophilised and powdered medications:
- The manufacture of lyophilised drugs has grown in both the pharmaceutical and biopharmaceutical sectors by around 13.5% per year over the last five years.2
- Of the top 100 drugs, 16% are lyophilised, according to BCC Research.3
- Due to the rapid growth in biologics, the percentage in this category is even higher, at 35% lyophilised, also according to BCC Research.
- Markets and Markets reports in their 2020 global forecast that more than half of injectable drugs will soon require lyophilisation,4 creating new challenges and opportunities for drug delivery.
WITH RISING USE COMES MORE CHALLENGES – ESPECIALLY WITH DIFFICULT-TO-MIX DRUGS
The growth with lyophilised injections is driven by several factors, each with its own challenges.
First is a rise in the use of lyophilisation as a preservation technique for biological products and drugs in the pharmaceutical industry.5 Lyophilised products have a much longer shelf life and thermal stability than products in liquid form.6 This is especially helpful for inherently shelf-unstable biologics – drug products on the cutting-edge of biomedical research. It also reduces the need for cold-chain management during shipping. Biologics, while one of the most effective means to treat several previously untreatable illnesses and conditions, are currently difficult to formulate and administer; their large molecular size often creates a highly viscous dose that can be easily degraded.
The use of depot injections is another factor driving an increased prevalence for powdered medications. Depot injections use extended-release medication formulations to enable long-acting drug dosing. A single depot injection could reduce a once-daily regimen down to bi-weekly, monthly or longer intervals. While designed to improve patient compliance and outcomes, depot formulations often have unique mixing challenges. They must be stored separately and then “suspended” in a liquid vehicle just prior to administration, a process that can also be difficult due to high viscosity and large-volume dosage requirements.
It is also increasingly common for companies to develop lyophilised formulations to get through their clinical trials and then reformulate a more user-friendly liquid version of the product for commercial sale. This process adds time and risk to their development programme as they test different drug delivery methods versus taking the lyophilised product all the way through approval to market.
When a lyophilised drug does make it into a patient’s treatment plan, the most common delivery solution for powdered and lyophilised medications continues to be a kit with two vials, multiple needles and a syringe.7 This approach requires several complicated steps to draw fluid from the diluent vial, dispense it into the powder container, shake or swirl vigorously, manually observe dissolution, attach a new needle, manually draw the combined ingredients and then inject. The process requires a substantial amount of time and medical training – although even with training, complicated procedures introduce or increase the possibility of human error and environmental impact, which can result in an incorrect dose, a reduction in a drug’s effectiveness or worse.
Current dual-chamber bypass cartridge technologies offer little improvement. While the two vials are integrated into a single cartridge containing both drug and diluent, drug delivery can be orientation-dependent and still reliant on vigorous shaking to ensure a full reconstituted dose. In addition, dual-chamber autoinjector manufacturing is often more complex, and the end-user patient experience is lacking – both of which leave a strong desire for something better.
Drug developers looking for devices must ensure they meet a diverse set of requirements, from balancing patient needs and compliance to the mixing complexities of effective drug delivery and a rapid path to market.
“Windgap addresses the critical challenges of delivering difficult-to-mix drugs with two dual-chamber reconstitution autoinjector platforms – its compact ANDIPen® and an LVDC autoinjector.”
INJECTING SIMPLICITY INTO COMPLEX DRUG DELIVERY DEVICES
One company in particular aims to simplify, automate and accelerate the drug delivery process for both pharmaceutical companies and the patients who depend on them.
Windgap Medical addresses the critical challenges of delivering difficult-to-mix drugs with two dual-chamber reconstitution autoinjector platforms – its compact ANDIPen® and a large-volume, dual chamber (LVDC) autoinjector. Each of these two wet/dry dual-chamber autoinjector platforms automates rehydration and administration, simplifies the reconstitution steps and allows the user to administer a dose in seconds.
One of the primary drivers of Windgap’s technologies is its focus on human factors engineering, which considers patient capabilities, limitations and lifestyle characteristics to develop products that bypass common delivery and manufacturing issues, all of which protect the medication, the patient and the outcome.
ANDIPen: Twice the Shelf-life, Half the Size
Windgap’s ANDIPen addresses significant yet unmet user needs within a competitive market by increasing portability, temperature stability, ease of use and shelf life for the medications it administers. The ANDIPen reduces the number of steps to two simple user operations: twist and inject (Figure 1).
- Cap twist and removal – A simple cap twist connects the diluent and powder chambers through a rotational valve. This simultaneously aligns two fluid channels to create a fluid pathway while releasing a spring that drives the diluent to interact with the powdered drug. This automatically rehydrates the correct dose and exposes the needle-shield-fired trigger. The interconnected chambers move inside the autoinjector to maximise the surface area and interaction between the two parts, speeding up dissolution.
- Injection – Depressing the needle shield initiates the automatic needle insertion into the skin and delivers the reconstituted medication. After device injection and removal, the needle shield extends to provide needle safety.
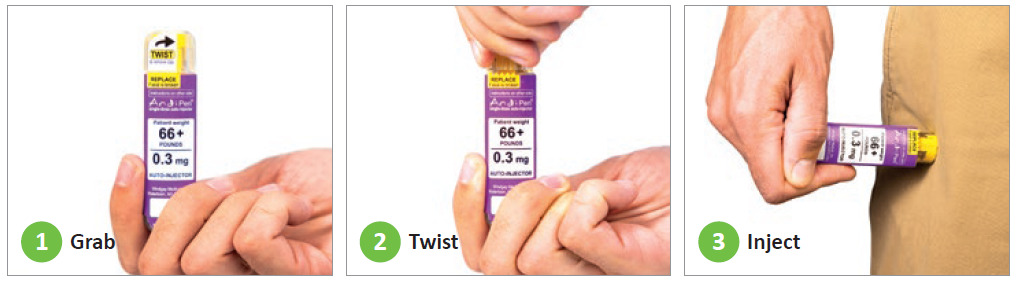
Figure 1: The ANDIPen reduces the number of steps to two simple user operations: twist and inject. Windgap Medical’s products are not commercially available or currently approved anywhere around the globe.
All of this occurs in just a few seconds – no shaking or swirling required. The ANDIPen platform can accommodate volumes of 0.3 mL or less, with a viscosity of up to 1 cP, with custom needle lengths and gauges for either subcutaneous or intramuscular delivery. The ANDIPen offers powerful and rapid device-controlled automixing capabilities within its volume and viscosity range – and is small enough to provide a patient with peace of mind in their pocket.
LVDC: Difficult-to-Mix Drugs at the Press of a Button, No Shaking Required
Capitalising on the success of its ANDIPen drug-delivery platform and with funding from the US National Institutes of Health, Windgap began developing an LVDC device to quickly and completely mix lyophilised drugs with viscosities up to and greater than 1000 cP and deliver dose volumes of up to 5 mL – opening the door for additional treatment areas in biologics, large molecule and lyophilise compatible medications.
The novelty of this device comes from its innovative primary drug container (PDC) configuration. Windgap’s PDC architecture uses two standard, off-the-shelf, single-chamber cartridges nested side by side and connected with Windgap’s novel proprietary mixing and delivery needle hub. The side-by-side nesting of the cartridges enables a short and more compact autoinjector using ISO standard cartridges that are compatible with industry-standard filling methods for powder and liquid products (Figure 2). The PDC architecture is simple and scalable, as well as able to accommodate a variety of cartridges size (1, 2.25, 3, 5 mL, etc.).
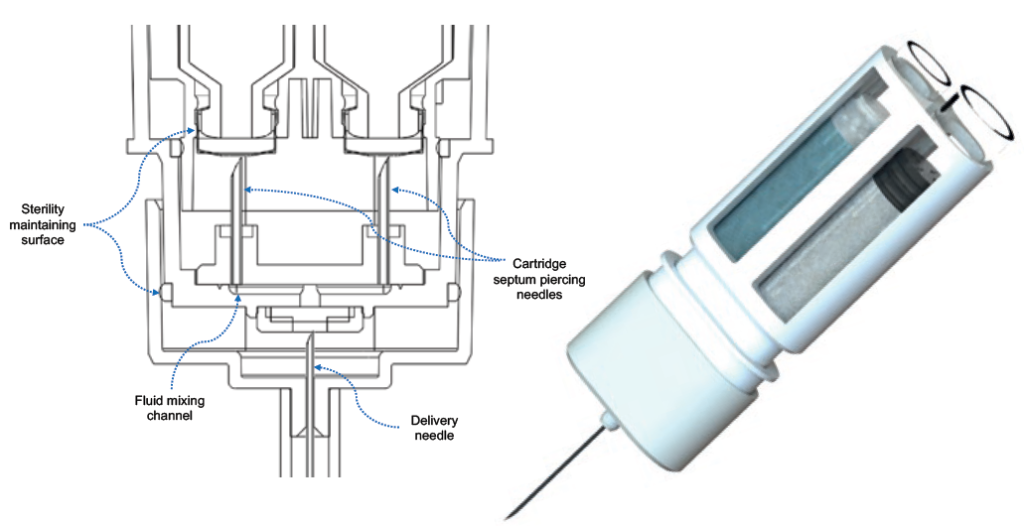
Figure 2: Left: Cut-away schematic of the mixing and delivery hub of the PDC. Right: Windgap’s side-by-side PDC with mixing and delivery hub.
“These proven platforms put Windgap in a prime position for collaboration with the pharmaceutical industry by simplifying drug delivery devices for any therapeutic application.”
When reconstitution is initiated, septum-piercing needles puncture both cartridges simultaneously. This dual puncture creates a closed fluidic connection between the two sides, transferring the liquid from its initial cartridge to the powdered drug cartridge for mixing. A single button press cycles fluid from one cartridge to the other. When the button is released, the fluid returns back to the original cartridge.
A stored gas energy source within the autoinjector governs the number of mixing cycles, using a regulated gas pressure to control the mixing/delivery force via a mixing valve. This technology can be adjusted to fit the number of cycle requirements, mixing pressure and delivery pressure to meet the drug and user needs.
Like Windgap’s ANDIPen device, the LVDC is orientation independent, so it can be used at any angle, in any environment. The cartridge size, mixing needle diameter, fluidic channel length and fluidic channel diameter are all customisable to serve a wide range of medications, including – and especially – difficult-to-mix drug products (Figure 3).
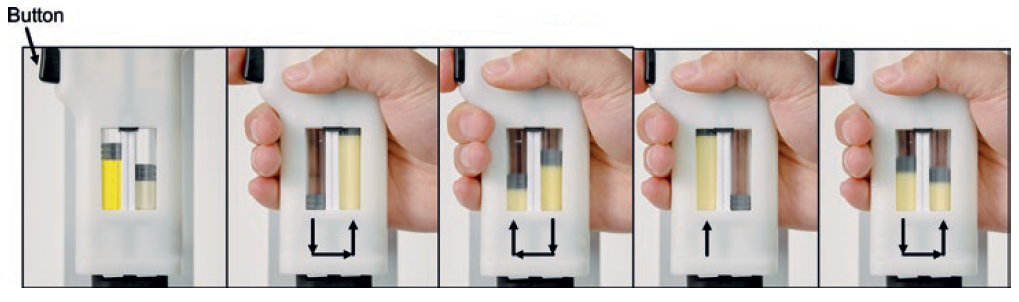
Figure 3: Demonstration of Windgap’s reciprocating autoinjector mixing a cyanide antidote drug product currently under development. Arrows indicate alternating fluid flow in the PDC between cartridges.
This Method of Reciprocating Mixing has Several Key Advantages
Internal studies have shown that this method of reciprocated mixing has decreased the mixing time for difficult-to-mix drugs from hours to seconds, increasing the rate of dissolution by an astounding 98% compared with conventional shaking and swirling methods.
Improved human factors and a reduction in mixing complexity reduces the potential for errors by the user. Drug delivery products that would normally be administered in the clinic can be self administered by the patient in the comfort of their own home.
Both of these proven platforms put Windgap in a prime position for collaboration with the pharmaceutical industry by simplifying drug delivery devices for any therapeutic application.
Windgap’s platforms drive early-phase innovation and speed to market while minimising and managing risk by taking a powdered product from clinical development all the way to commercial production, often faster and at a lower cost than developing a stable liquid formulation midway through R&D.
CONCLUSION
As the injectables market continues to skyrocket, the demand for simple, automated and easy-to-use reconstitution devices will continue to rise, especially with regard to lyophilised medications. Both of Windgap’s platforms have been designed to be compliant with emergency-use reliability requirements. The company’s technology platforms continue to rise to the challenge, with a team of experts ready to collaborate on innovative pharmaceutical solutions built with patients in mind.
Windgap’s first device programme has been globally licensed to, and is being commercialised by, ALK Abelló, a Danish pharmaceutical company located in Hørsholm. This programme uses Windgap’s ANDIPen for the delivery of reconstituted adrenaline (epinephrine).
Windgap Medical’s products are not commercially available or currently approved anywhere around the globe.
REFERENCES
- “Generic Injectable Market: Growing Focus on the Development of Cost-Effective Treatment for Rare Diseases is Major Factor Driving the Industry Growth”. Research Report, Zion Market Research, Jul 14, 2021.
- “Lyophilization Services for Biopharmaceuticals Market Forecast to 2027 – COVID-19 Impact and Global Analysis by Service Type, End User, and Geography”. Research and Markets, Jul 2020.
- “Lyophilized Injectable Drugs Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2018-2026”. Research and Markets, Feb 2019.
- “Freeze-Drying/ Lyophilization Market by Type (Tray, Shell, Manifold), Scale of operation (Industrial, Lab, Pilot), Application (Food, Pharma & Biotech), Accessories (Loading & Unloading, Monitoring, Vacuum Systems, Drying Chambers) – Global Forecast to 2025”. MarketsandMarkets, Oct 2020.
- Mirasol F, “Lyophilisation Presents Complex Challenges”. BioPharm Int, 2020, Vol 33(1), pp 22–24.
- “Guide to Inspections of Lyophilisation of Parenterals”. US FDA, Nov 2014.
- “Let’s Mix Things Up! Innovations in Reconstitution Technologies”. Emergo by UL, Jun 13, 2021.

