To Issue 142
Citation: Roested J, “Low-Complexity, Easy-To-Use Wearable Injection Platform”. ONdrugDelivery, Issue 142 (Feb 2023), pp 32–35.
Jesper Roested discusses the increasing prevalence of biologics formulated for large-volume injections in drug development and how wearable devices, including Subcuject’s offering, may be a better solution for delivering these drugs than scaled-up conventional autoinjectors.
“To address the challenges of conventional autoinjectors and electromechanical wearable injectors, Subcuject has developed a platform of wearable injection devices based on osmosis (salt and water) and a spring-driven injector.”
PUSHING AUTOINJECTORS ABOVE 1 ML
During recent years, drug development pipelines have come to include biologics for subcutaneous delivery in volumes above 1 mL. This development has been driven by factors including a push towards at-home use and increased convenience. Previously, the general consensus was that 1 mL could be delivered quickly, but anything above 1 mL would require a slow injection. As such, autoinjectors were limited to 1 mL until the first 2.25 mL autoinjector was launched in 2020.
Single-use, handheld autoinjectors have now become a successful method for administering biologics up to 2.25 mL, and a variety of autoinjectors are available from a large number of suppliers. A number of 5 mL handheld autoinjectors are now also in development, responding to market needs for increasing demand for higher volume delivery.
Delivering volumes larger than 2 mL in a conventional handheld autoinjector may require a permeation enhancer, as well as either very fast injection or for the injector to be held steady for an extended period of time. It has been claimed that patients can hold a handheld autoinjector in place for 60–70 seconds.1,2 However, there may be a risk of premature removal, resulting in wet injections, if the hold time is increased. As such, scaling up the size of conventional handheld autoinjectors may not be the ideal solution for larger-volume injections.
The field of larger-volume wearable injectors was established in 2016 with the launch of Repatha (evolocumab, Amgen, CA, US) in the Pushtronex 3.5 mL wearable injector. Since then, other drugs formulated for wearable injectors have entered the drug development pipeline. It is expected that a number of new drugs, currently in clinical trials, will be approved as combination products in wearable injectors over the next few years.
“Scaling up the size of conventional handheld autoinjectors may not be the ideal solution for larger volume injections.”
Wearable injectors were developed to eliminate the need to hold an autoinjector steady when delivering larger-volume drugs without permeation enhancers, enabling much slower injection. It is a technical challenge to generate a slow release of energy from traditional drug delivery device power sources, such as springs. As such, many of the wearable injectors currently in development are based on electromechanical solutions where energy release is electronically controlled, resulting in complex devices that also may be less acceptable for disposal after single use.
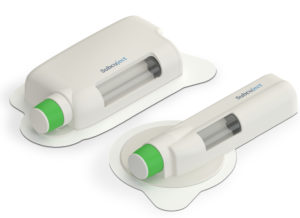
Figure 1: Subcuject’s wearable platform devices.
SUBCUJECT’S WEARABLE AUTOINJECTOR PLATFORM
To address the challenges of conventional autoinjectors and electromechanical wearable injectors, Subcuject has developed a platform of wearable injection devices based on osmosis (salt and water) and a spring-driven injector (Figure 1). The devices share the same user interface, are easy to use and are intended as prefilled single use devices. Both are characterised by low technical complexity. Furthermore, both devices use standard primary packaging in the form of a glass cartridge with a standard septum and a conventional rubber compound for the plunger.
In its current configuration, the osmotic wearable injector holds up to 5 mL and could be adapted to hold 10 mL or more, with an injection rate of around 1 mL per minute. The benefit of an osmotic actuator is that energy is generated over time – no slowdown is needed. Furthermore, as the device platform does not include any electronics, it is better suited for disposal after single use than an electromechanical device.
“Subcuject has a partnership with LTS Lohmann Therapie-Systeme AG for the development and commercialisation of these wearable platform devices.”
In its current configuration, the spring driven wearable injector holds 3 mL and can be adapted to hold more than 5 mL. When attached to the skin, longer holding time is not an issue. It is intended for medium flow rates below 5 mL/minute. The configuration with the needle inside of the housing and subsequent needle retraction means that the user will never see the needle in the intended use case.
The 5 mL osmotic wearable bolus injector (Figure 2) has the following features:
- Prefilled
- Single use
- Small size
- Makes no noise
- Uses a glass cartridge and standard primary packaging components
- One button activation
- Visual, audible and tactile end-of-dose confirmation
- Automatic needle retraction
- No electronic components.
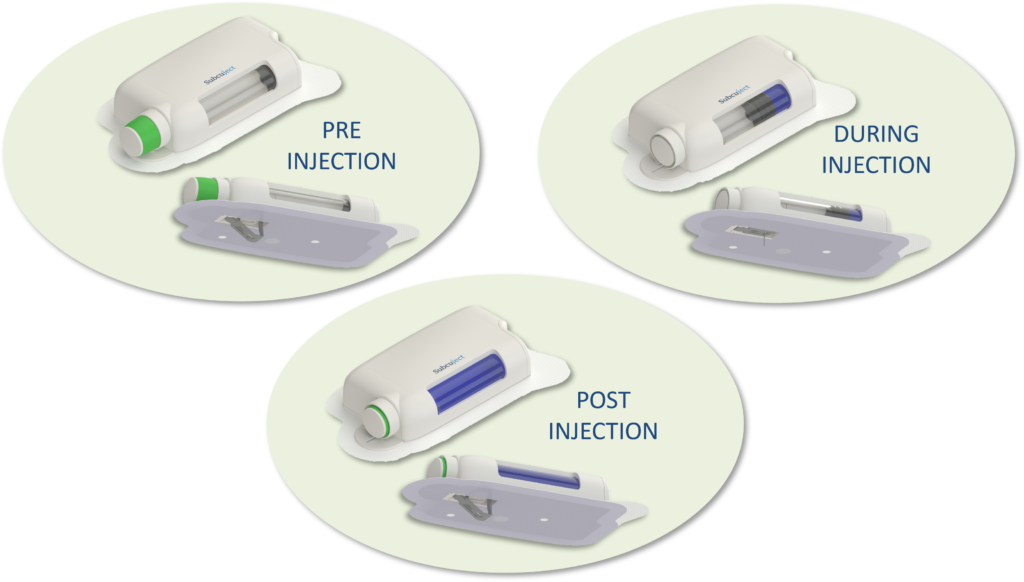
Figure 2: Use sequence for the osmotic wearable bolus injector.
The 3 mL spring-driven wearable injector (Figure 3) has the following features:
- Prefilled
- Single use
- Small size
- Uses a glass cartridge and standard primary packaging components
- One button activation
- Visual, audible and tactile end-of-dose confirmation
- Automatic needle retraction
- No electronic components.
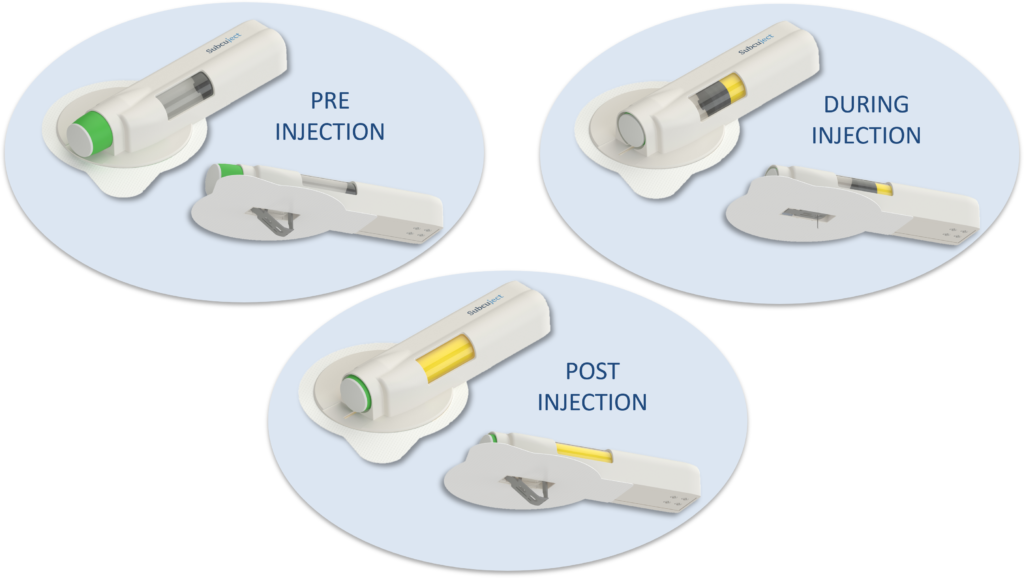
Figure 3: Use sequence for the wearable autoinjector.
Subcuject has a partnership with LTS for the development and commercialisation of these wearable platform devices. LTS is a leading pharmaceutical technology company that develops and manufactures innovative drug delivery systems, such as transdermal datches, oral thin films and micro-array patches.
REFERENCES
- Jost R, “The New YpsoMate™ 5.5 – Taking Handheld Self-Injection Beyond Volumes of 2 mL”. ONdrugDelivery, Issue 138 (Oct 2022), pp 8–11.
- Calderwood G, Ganea R, Fuensalida Pantig G R, “Enlarging the Volume of Autoinjectors: Traversing Injection Boundaries”. ONdrugDelivery, Issue 138 (Oct 2022), pp 26–32.

