To Issue 131
Citation: Pirera P, “Manufacturing DPIs: an Engineering Perspective”. ONdrugDelivery, Issue 131 (Apr 2022), pp 71–74.
Pietro Pirera discusses the optimal process parameters for microdose dry power inhalers achieved by dosator technology.
“Patients and pneumologists are now increasingly focusing
on convenience and ease of use, favouring a compact design.”
When developing new dry power inhalers (DPIs), industrial manufacturing aspects must be considered from the very beginning to speed up the scale-up and optimisation of the final manufacturing process, as well as to achieve a more efficient and cost-effective production. Precise microdosing, weight control and ease of device assembly are all issues that must be faced at an early stage.
IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and assembly. Direct weight control performed in line on each single capsule or device, both before and after filling, for example. No mechanical powder compression – for improved airway intake. And accurate microdosing, as well as automatic feedback and adjustment.
This study investigates optimal process parameters for microdose DPIs achieved by dosator technology. The study proves that a major advantage of using this technology for processing DPIs is that the dosators can be accurately adjusted without any need to compress or aspirate the powder. Maintaining the free-flowing properties of the dispensed powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced, thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.
INTRODUCTION
In 1948, the first commercial DPI was launched on the market. This first technology seems archaic by today’s standards: a deep inward breath would cause a ball to strike a cartridge containing powder and shake the powder into the airstream. Since then, changes in the drug delivery market and regulatory pressures have driven DPI innovation. It is estimated by the WHO that, worldwide, some 300 million people suffer from asthma and 240 million people suffer from chronic obstructive pulmonary disease (COPD). DPIs represent 50% of the total asthma/COPD market by value worldwide.
The latest patient-focused studies using DPIs indicate that the expectations regarding this technology have evolved. Patients and pneumologists are now increasingly focusing on convenience and ease of use, favouring a compact design. Indeed, DPIs have shown great promise in their ability to deliver drugs reliably and effectively, and novel designs can ensure that future cost, compliance and safety challenges are overcome.
Some of the performance characteristics essential to DPIs are related to dose delivery, fine particle fraction content and performance levels at varying airflows. These characteristics can differ from one powder formulation to another, and some fine tuning of either device or formulation– or a combination of both – may be necessary to achieve optimal performance. Microdosing DPIs take this challenge to extremes. IMA Active (Bologna, Italy) and Medochemie (Limassol, Cyprus) have joined forces to achieve optimal microdose DPIs by combining dosator technology and the direct net weight control in a tabletop device and in an industrial-production-scale capsule filler.
Some of the performance characteristics essential to DPIs are related to dose delivery, fine particle fraction content and performance levels at varying airflows.”
STUDY AIM
The aim of the study was to explore the best process parameters to achieve the 5.5 mg dose of a powder mix, including a first lactose type as carrier and a second one (4% in concentration by weight) of microfine lactose as an API simulator. The process was carried out first in a tabletop capsule-filling device (Minima, IMA) and then scaled up to an industrial-production-scale capsule-filling machine (Adapta with 100% gravimetric net weight control, IMA). Two types of lactose were compared from different suppliers.
MATERIALS
Components of the tested formulations are:
A. Blend of placebo powder composed of InhaLac 251 (Meggle, Germany) and 4% in concentration of Lactochem microfine lactose (DFE, Germany).
B. Blend of placebo powder composed of Respitose (DFE, Germany) and 4% in concentration of Lactochem microfine lactose.
For the execution of the tests, HPMC capsules size 3 were used (Table 1).

Figure 1: Minima tabletop capsule filler.
METHODS
The Minima and Adapta capsule fillers are designed to process microdose DPIs. Minima (Figure 1) is a tabletop capsule filler designed to dose solid products in hard capsules. Extremely precise and with a single dosator, Minima can be equipped with the same dosing devices applied on IMA production machines. Minima comes factory preset for use with DPIs, thus being an efficient system for inhalation product research, development, optimisation and protocol validation.

Figure 2: Adapta high-speed capsule filler.
The Adapta capsule-filling machine (Figure 2) covers medium and very high-speed production requirements and features exceptional design flexibility. Fitted with the 100% gravimetric net weight control (Figure 3), Adapta ensures maximum dosing precision and reliability even at very high speed. On the Minima machine, the target dosages of 5.5, 15 and 25 mg were achieved with both formulations.
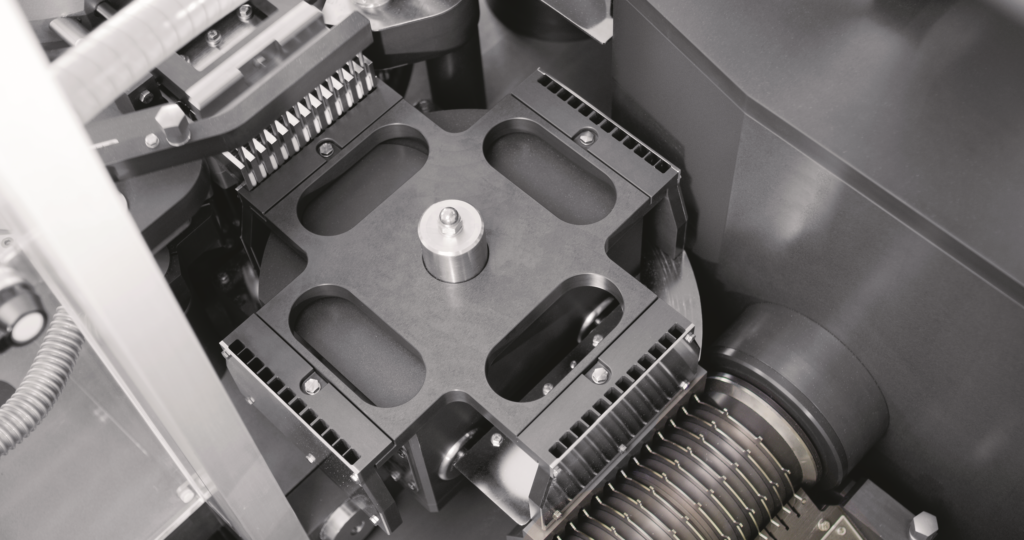
Figure 3: Adapta 100% gravimetric net weight control.
“A major advantage of using dosator technology for processing low-dose DPIs is that the system can dose very small amounts of powder into capsules.”
Each lot consisted of 20 samples. Reliability and consistency were assessed by replicating the acquisitions three times for each lot. The second step of the study was to scale up the experience gained on the benchtop machine to the production equipment. Since the target dose was 5.5 mg, the main work was concentrated on this target with both preparations. A total of 100,000 capsules were produced with Adapta with 100% gravimetric net weight control for each blend.
To determine the net weight of the samples dosed with Minima, the macro-analytical electronic scale Sartorius was used. Weighing range: 220 g, accuracy: 0.1 mg. To check the net weight of the samples dosed with the capsule-filling machine, the total filling control system of Adapta was used.
| Powder mix | Bulk density (g/mL) |
Tapped density (g/mL) |
Car Index (%) |
Loss on drying (%) |
| InhaLac 251 + 4% Lactochem microfine lactose |
0.593 | 0.780 | 23.9 (poor flowability) |
0.04 |
| Respitose SV003 + 4% Lactochem microfine lactose |
0.658 | 0.812 | 18.9 (fairly good flowability) |
0.08 |
Table 1: Technological characteristics of the two kinds of powder mixes – InhaLac 251 and Respitose with 4% Lactochem microfine lactose each.
| Average net weight (mg) |
Doser internal diameter (mm) |
Dosing chamber position (mm) |
Powder layer height (mm) |
Min-max weight sample deviation (mg) | Tolerance obtained (%) |
Relative Standard Deviation (%) |
| 25.9 | 3.0 | 4.1 | 15 | 1.48 | +3.3 to -2.3 | 2.03 |
| 14.6 | 2.5 | 3.7 | 15 | 0.74 | +1.6 to -3.4 | 1.48 |
| 5.4 | 2.0 | 2.4 | 10 | 0.70 | +8.0 to -6.1 | 3.0 |
Table 2: InhaLac 251 + 4% Lactochem microfine lactose, Minima trials.
| Average net weight (mg) |
Doser internal diameter (mm) |
Dosing chamber position (mm) |
Powder layer height (mm) |
Min-max weight sample deviation (mg) | Tolerance obtained (%) |
Relative Standard Deviation (%) |
| 25.5 | 3.0 | 4.2 | 15 | 1.75 | +3.4 to -3.4 | 2.24 |
| 14.6 | 2.5 | 3.8 | 15 | 0.46 | +1.5 to -1.6 | 0.90 |
| 5.5 | 2.0 | 2.2 | 10 | 0.67 | +4.8 to -8.1 | 2.9 |
Table 3: Respitose SV003 + 4% Lactochem microfine lactose, Minima trials.
| Lactose kind | Average net weight (mg) |
Doser internal diameter (mm) |
Dosing chamber position (mm) |
Powder layer height (mm) |
Relative Standard Deviation (%) |
Machine speed (caps/h) |
| InhaLac 251 + + 4% Lactochem microfine lactose |
5.5 | 2.0 | 2.13 | 10 | 2.52 | 85,000 |
| Respitose SV003 + + 4% Lactochem microfine lactose |
5.5 | 2.0 | 2.18 | 10 | 2.52 | 85,000 |
Table 4: Powder mixtures Adapta with 100% gravimetric net weight control trials.
EXPERIMENTAL PART
In Tables 2 and 3, the experiments of the Minima first screening are reported, including machine setting, real weight achieved, tolerances, range between minimum and maximum sample weight obtained and relative standard deviation.
Table 4 summarises the final results of the Adapta with 100% gravimetric net weight control: the powder mixtures and machine settings are reported, including real weight achieved and relative standard deviation for an easy evaluation.
Figures 4 and 5 show the net weights of all 24 dosators of the Adapta with 100% gravimetric net weight control for both powder mixes.
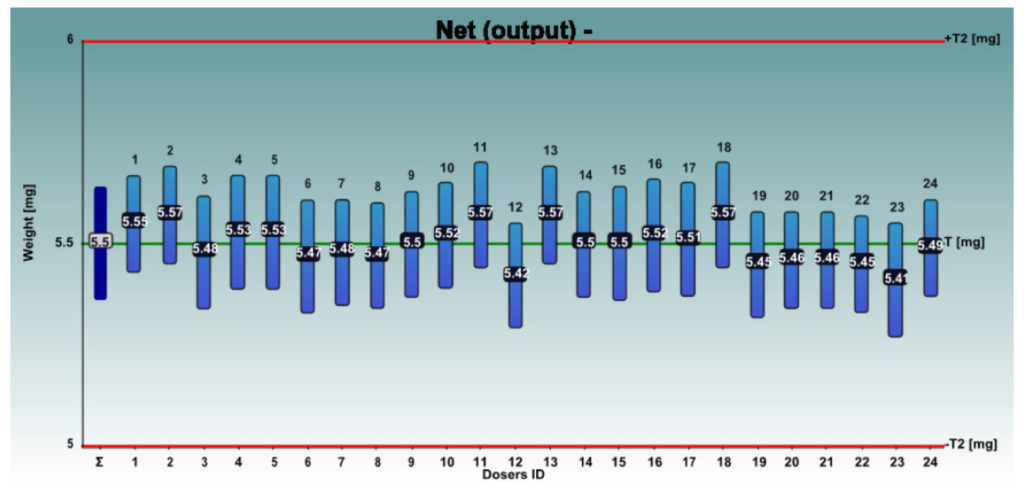
Figure 4: InhaLac 251 + 4% Lactochem microfine lactose, behaviour of the 24 dosators on Adapta with 100% gravimetric net weight control.
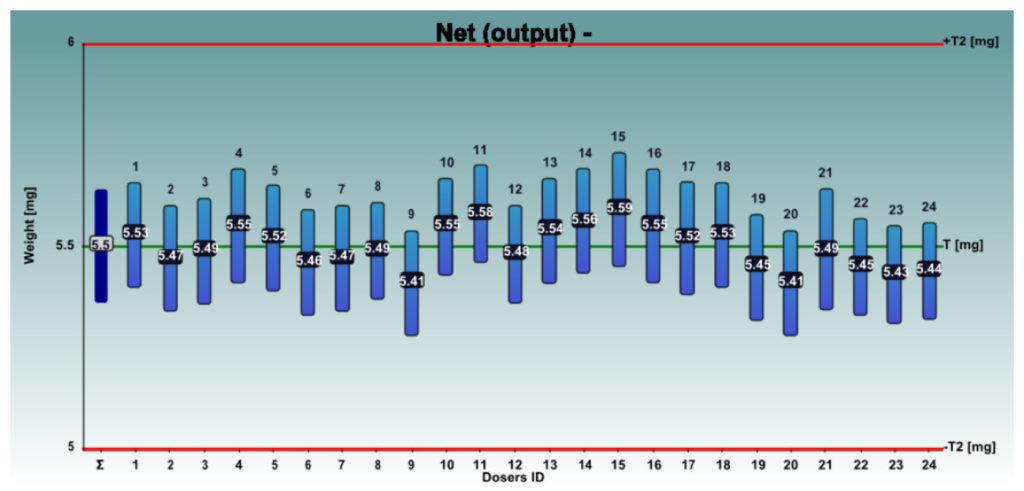
Figure 5: Respitose SV003 + 4% Lactochem microfine lactose, behaviour of the 24 dosators on Adapta with 100% gravimetric net weight control.
RESULTS
The tests on Minima demonstrated that both formulations gave good results in terms of workability and tolerance obtained. No significant differences were observed by the operator. Both formulations demonstrated good behaviour, even on the machine Adapta with 100% gravimetric net weight control: no seizing and no empty capsules produced. It was confirmed that for 5.5 mg dosing, the range between the minimum and maximum weight value in the tabletop capsule filler was always lower than 1 mg. The results obtained once formulations were tested in the production-scale capsule-filling machine were even better: for both formulations, the relative standard deviation was confirmed below 3%.
DISCUSSION AND CONCLUSION
As proven by this study, a major advantage of using dosator technology for processing low-dose DPIs is that the system can dose very small amounts of powder into capsules. This powder-dosing technology does not require powder compaction to transfer the powder to the capsule. This ensures that the powder within the capsule is less likely to form aggregates and is maintained as a free-flowing powder.
Maintaining the free-flowing properties of the dispensed powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced, thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.

