To Issue 160
Citation: Gurruchaga L, “Navigating the Transition: Exploring Alternatives to TiO2 in Pharmaceutical Formulations”. ONdrugDelivery, Issue 161 (May/Jun 2024), pp 6–11.
Lucía Gurruchaga discusses the push to develop an alternative opacifier and white colourant to titanium dioxide in the wake of the European Commission’s announcement that the additive may be banned in medicinal products, presenting data from recent testing on Qualicaps’ own novel alternative.
Titanium dioxide (TiO2), also known as E-171, is an inert, naturally occurring material that is produced in two main different forms. The primary form, which represents over 98% of total production, is the pigment grade. The pigmentary form is used in applications that require white opacity and brightness, which TiO2 is ideal for due to its excellent light scattering properties. Some of these applications are paints, coatings, plastics, inks, foods, medicines and toothpastes. The other form is an ultrafine nanomaterial product. This form is selected when different properties, such as transparency and maximum UV light absorption, are required – for example, in cosmetic sunscreens.1
“TiO2 stops light transmission through a film by acting as an opacifier. This property has been widely used in the pharmaceutical and consumer healthcare industries as a white pigment and opacifier for oral solid dosage forms, such as tablets and hard and soft capsules.”
TiO2 stops light transmission through a film by acting as an opacifier. This property has been widely used in the pharmaceutical and consumer healthcare industries as a white pigment and opacifier for oral solid dosage forms, such as tablets and hard and soft capsules. Approximately 91,000 human medicinal products and 800 veterinary medicinal products contain TiO2 in the EU, according to EU trade associations.2 However, in recent years, the use of this excipient has been questioned due to safety concerns.
REGULATORY BACKGROUND AND CURRENT STATUS
In 2015, the European Commission (EC) requested that the European Food Safety Authority (EFSA) evaluate the safety of E-171 as a food additive. In 2016, the EFSA concluded that no real concerns existed about the continued safe use of E-171 but recommended that more tests be performed to complete the toxicological studies. In June 2018, the French National Assembly enacted a temporary suspension of the use of E-171 as a food additive. No other EC members supported this position, deciding rather to wait for all ongoing studies to be completed before taking further action.
In May 2021, the EFSA published a safety assessment of E-171 as a food additive based on scientific evidence considered by the panel to be reliable, including data obtained with TiO2 nanoparticles and data from an extended one‐generation reproductive toxicity study. Genotoxicity studies showed that TiO2 particles have the potential to induce DNA strand breaks and cause chromosomal damage, but not gene mutations. No clear correlation was observed between the physicochemical properties of TiO2 particles and the outcome of either in vitro or in vivo genotoxicity assays. Therefore, based on all the available evidence, a concern for genotoxicity could not be excluded and, given the many uncertainties, the panel concluded that E-171 can no longer be considered safe when used as a food additive.3 The EFSA neither identified nor recommended any new studies to clarify the genotoxicity concern and other remaining uncertainties.
In September 2021, the EMA, in response to a request from the EC, provided a scientific analysis on the technical purpose of the use of TiO2 in medicinal products, the feasibility of replacement and possible timeframes for alternatives. In its conclusions, the EMA stressed that, from a technical point of view, it should be possible to find alternatives to replace TiO2 both as a colourant and for other uses. The EMA highlighted the need to carefully assess alternatives to ensure their compatibility with the various components of individual pharmaceutical products. Furthermore, the EMA concluded that it was difficult to recommend a precise transition period timeframe for the replacement of TiO2 in medicinal products, considering the time needed to reformulate each individual product.2
Based on the EMA scientific analysis, and in order to avoid shortages of medicinal products that could have impacts on public health, Commission Regulation (EU) 2022/63 stated that TiO2 should remain provisionally on the list of authorised additives to allow its use in medicinal products as a colourant, pending the development of adequate alternatives to replace it, while ensuring the quality, safety and efficacy of the medicinal products concerned. However, the EC also encouraged the pharmaceutical industry to accelerate the research and development of alternatives to be used as a replacement for TiO2 in medicinal products, and to submit the necessary variation to the terms of the marketing authorisations concerned.4
“The EC has committed to review the necessity to maintain TiO2 or otherwise delete it from the list of food additives for exclusive use as a colourant in medicinal products within three years from the date that the regulation entered into force.”
Finally, the EC has committed to review the necessity to maintain TiO2 – or otherwise delete it from the list of food additives for exclusive use as a colourant in medicinal products – within three years from the date that the regulation entered into force. This review was set to be based on an updated assessment of the EMA, expected to be published in April/May 2024. It should account for the progress made during this period in developing alternatives for TiO2 in medicinal products both for new and existing products. Possible impacts on quality, safety and efficacy have to be assessed and avoided. As of writing, there has not been an update either from the EMA or from the EC in this regard.
In May 2023, the US FDA submitted a Citizen Petition from the Environmental Defense Fund entitled “Request To Revoke Color Additive Listing for Use of Titanium Dioxide in Food”, which is still open. In November 2024, the Joint Expert Committee on Food Additives (JEFCA), formed by the WHO and the Food and Agriculture Organization (FAO), issued an assessment of the health impacts of TiO2 as a food additive. After reviewing the available scientific literature, the JEFCA determined that more research was needed to address the current uncertainty about the distribution of TiO2 particle sizes.5
At present, there is still not enough data to issue a final conclusion on TiO2. Countries such as China, the US and Brazil continue to carry out their evaluations.
MARKET SITUATION
During this period, the pharmaceutical industry has faced several challenges in the transition from TiO2 to alternative opacifiers. As mentioned prior, TiO2 has been a widely used excipient in the pharmaceutical industry for more than 50 years. It is present in the majority of pharmaceuticals, in the coating of tablet films for capsule shells, as well as packaging materials. Its extensive use is due to its critical functions, which result from the following properties:
- Inert substance: TiO2 is of particular benefit because it does not impact the properties of APIs or excipients. Being an unreactive ingredient, it is a common substance for medicines because it is well tolerated.
- Consistent homogeneous colouring: TiO2 enables a consistent colour scheme and plays an important role as an opacifier. Consistent colouring of medicines plays a significant role in the recognition of a medicine to allow for differentiation for the patient, who needs to be able to readily distinguish between multiple medicine types, which can have a direct impact on therapeutic adherence and patient safety. In addition, colour variations may give false indication to the user that the product has degraded or is not efficacious.
Overall, TiO2 contributes to developing a robust dosage form, protecting APIs, ensuring shelf-life stability and, therefore, securing the safety and efficacy of pharmaceuticals for longer periods.6
As pointed out by the “Use of titanium dioxide as excipient in human medicines. Industry feedback to QWP Experts/EMA Questions ” survey, investigations into alternatives should identify an excipient with comparable properties regarding opacity, transmittance, water solubility and particle size, leading to the same uniform film quality as TiO2. The alternative excipient must be safe for the patient in all aspects and compatible with the medicinal products in question.
The replacement of TiO2 presents an important multifactorial challenge. Besides demonstrating that the replacement will have the same or sufficient similar performance, a seamless supply in line with the required quality standards for pharmaceuticals has to be ensured.
One of the alternatives to TiO2 that has been identified during this period is calcium carbonate (CaCO3). However, it can’t provide the same degree of opacity and whiteness as TiO2. Additionally, as excipients, carbonates need to be included at a higher concentration than TiO2, which can have a negative impact on the film coatings by reducing film strength or increasing capsule brittleness. For light-sensitive drugs, it may also cause a higher rate of degradation.
“Qualicaps has successfully validated a new alternative formulation with a new opacifying and whitening agent for TiO2-free HPMC and gelatin hard capsules, which is now commercially available as Quali-V® TiO2-free and Quali-G™ TiO2-free.”
QUALICAPS SOLUTION
Qualicaps Europe has played a pioneer role in recent years in developing a new TiO2-free formulation that can overcome these challenges. The company’s research and development work has been focused on developing a new alternative that complies with safety, quality and regulatory standards. As a result, Qualicaps has successfully validated a new alternative formulation with a new opacifying and whitening agent for TiO2-free hydroxypropyl methylcellulose (HPMC) and gelatin hard capsules, which is now commercially available as Quali-V® TiO2-free and Quali-G™ TiO2-free. Some of the scientific data derived from the development work, compiled in the corresponding capsule product technical dossiers, are presented below.
Safety-Related Results
Particle Size Distribution
Figure 1 shows the results obtained from the particle size distribution (PSD) analysis performed with Quali-V® TiO2-free and Quali-G™ TiO2-free capsules. These results demonstrate the absence of nanomaterials in both formulations containing the new opacifier.
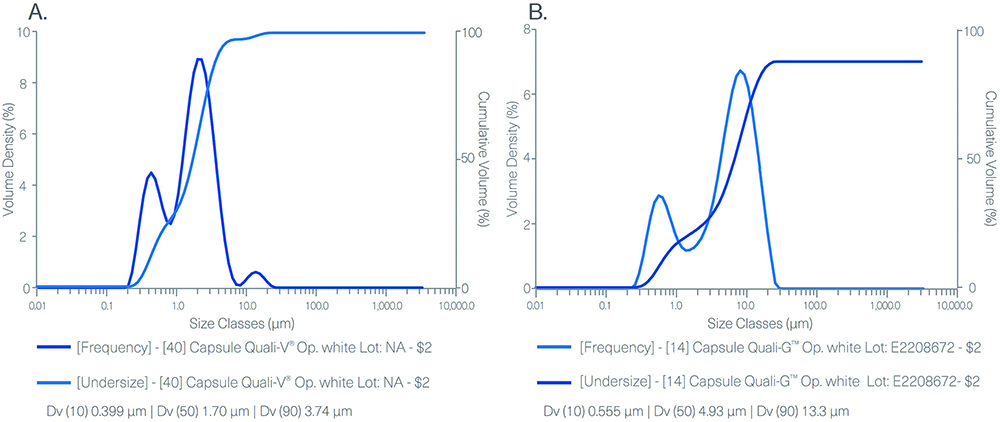
Figure 1: PSD result for Quali-V® TiO2-free capsule (A) and Quali-G™ TiO2-free (B) measured by laser diffraction in a Mastersizer 3000 (Malvern Panalytical, Malvern, UK) apparatus.
Quality-Related Results
Opacity
For Quali-V® capsules, the opacity was analysed by comparing different formulations containing different types of opacifiers and concentrations. The results show that the highest opacity is obtained with the capsules formulated with TiO2. Quali-V® capsules formulated with the new opacifying agent show the second highest opacity. The lowest opacity is obtained with the Quali-V® capsules formulated with CaCO3 (Figure 2).
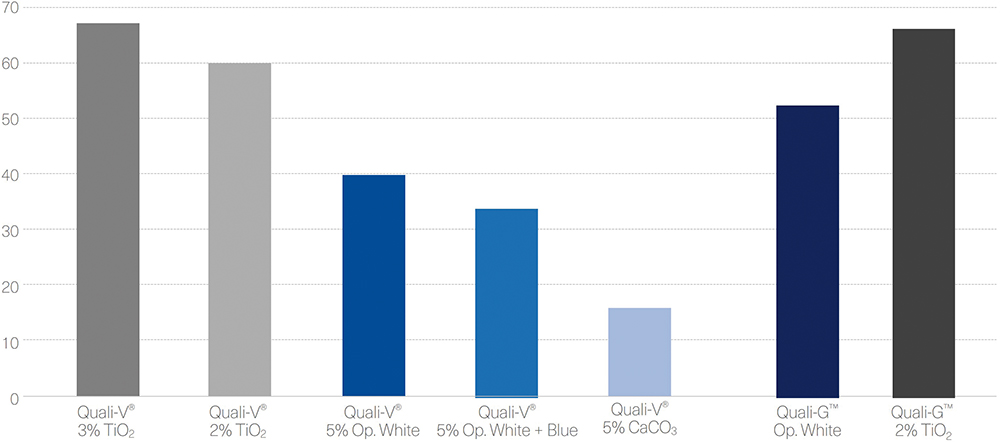
Figure 2: Opacity results of Quali-V® and Quali-G™ formulated with different opacifying agents, measured with a Lab Scan® XE (HunterLab, VA, US).
For the Quali-G™ capsules, two formulations were compared – Quali-G™ containing 2% of TiO2 and Quali-G™ formulated with the new opacifier (Figure 2).
Transmittance
Figure 3 shows the results obtained from the transmittance test performed with Quali-V®, using different opacifying agents. The results show that there is a big difference in transmittance between the Quali-V® formulated with CaCO3 and the other formulations. For Quali-G™, transmittance is slightly higher when formulated with the new opacifier, but in line with the results obtained with HPMC.
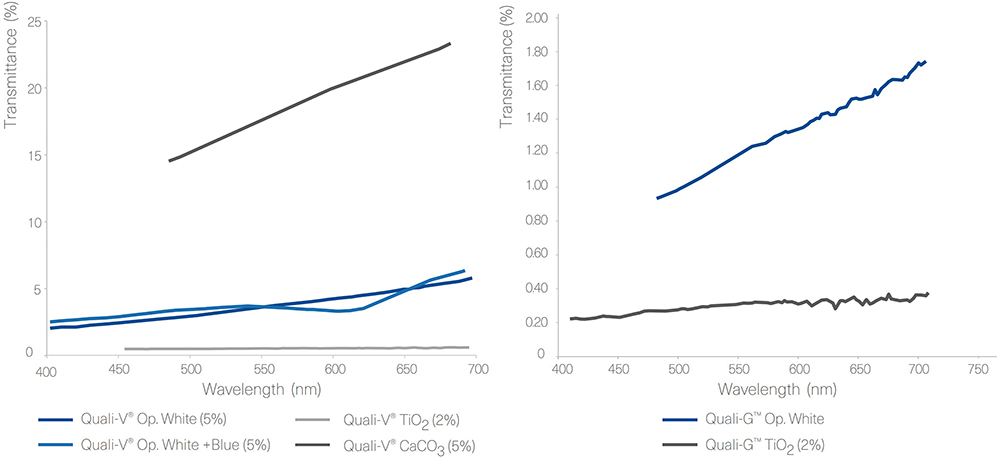
Figure 3: Transmittance results of Quali-V® and Quali-G™ formulated with different opacifying agents, analysed using a Cary 60 UV-Vis spectrophotometer (Agilent, CA, US).
Disintegration and Dissolution
Figure 4 shows a comparison between the dissolution profiles for Quali-V® TiO2-free capsules and Quali-V® with TiO2 at pH 1.2 and pH 6.8. All capsules tested complied with the specifications described in European Pharmacopoeia (EP) Ed 11.0, monograph 2.9.3, “Dissolution Testing for oral dosage forms”, and Chapter 5.17.1, “Recommendations on Dissolution Testing for immediate release dosage forms”, in which the acceptance criteria is stated as no less than 80% of the API dissolves in less than 45 minutes. Quali-V® TiO2-free and Quali-V® with TiO2 dissolution profiles are comparable at both pH levels.
Disintegration tests of empty Quali-V® TiO2-free capsules were performed following the analytical method described in the chapter 2.9.1 “Disintegration of tablet and capsule” of the EP 11th Edition. All the batches analysed met the specification of no more than 15 minutes.
For the gelatin capsules, a dissolution profile comparison was performed between Quali-G™ TiO2-free and Quali-G™ with TiO2 at pH 1.2 and pH 6.8 (Figure 5). All dissolution profiles are comparable and comply with the specification of immediate release dosage forms described above, since more than 80% of the API is dissolved in less than 45 minutes.
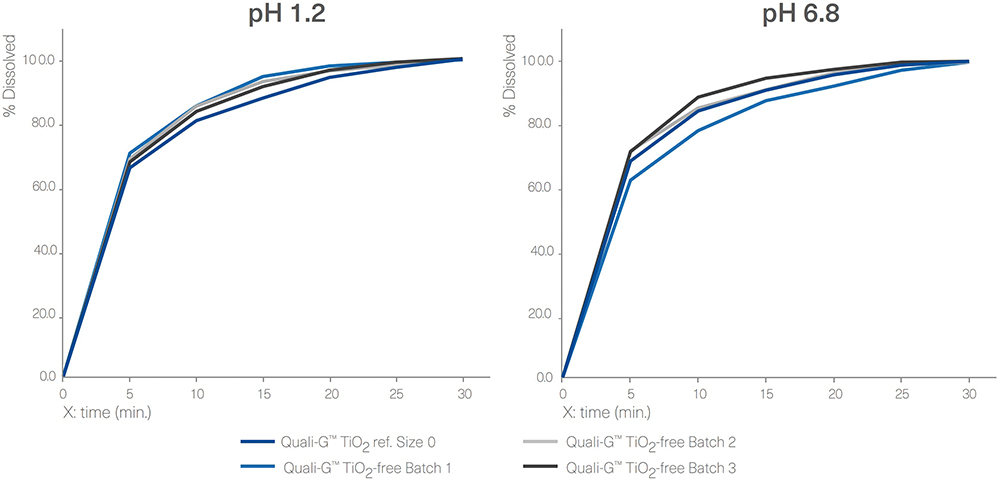
Figure 4: Dissolution profiles of four different batches of Quali-V® formulated with the new opacifier (Quali-V® TiO2-free) compared with Quali-V® formulated with TiO2 (Quali-V® TiO2) at pH 1.2 and 6.8 (capsule fill formulation: acetaminophen 20%, lactose 80%; dissolution test method: paddle at 50 rpm).
The results obtained from the disintegration test of empty Quali-G™ TiO2-free capsules showed a disintegration time of no more than 15 minutes, complying with the acceptance criteria. The analytical method applied was the same as described above for the HPMC disintegration tests.
Stability Studies
Stability studies were performed according to ICH Topic Q1A (R2), “Stability testing of new Drug Substances and Products”, and ICH Q1E, “Evaluation of Stability Data”, for both Quali-V® TiO2-free and Quali-G™ TiO2-free. Stability-indicating parameters, such us brittleness, dimensions, weight and loss on drying, were tested at every time point for long-term, intermediate and accelerated stability. All the results obtained to date have been satisfactory and compliant with the acceptance criteria. The current shelf life for both Quali-V® TiO2-free and Quali-G™ TiO2-free products is 24 months. The ongoing long-term stability study will continue up to 60 months.
CONCLUSION
The pharmaceutical industry is facing a big challenge due to the EC’s announcement of a potential ban on using TiO2 as an excipient in medicinal products. As encouraged by the EC, hard capsule manufacturers have been working for the last few years to offer an alternative solution to this widely used opacifier and white colourant. As of writing, the EMA has not announced any official decision derived from the assessment expected by April/May 2024, on which the EC will base its final decision on regulating TiO2 for medications.
Qualicaps has developed and launched a new alternative solution that complies with safety and quality standards, providing excellent appearance and performance, for both HPMC and gelatin capsules.
REFERENCES
- Beetsma J, “Transparent Inorganic Pigments: Titanium Dioxide and Iron Oxide”. Ultrus Prospector, Aug 2022.
- “Final feedback from European Medicine Agency (EMA) to the EU Commission request to evaluate the impact of the removal of titanium dioxide from the list of authorised food additives on medicinal products”. EMA, Sep 2021.
- “Safety assessment of titanium dioxide (E171) as a food additive”. EFSA, May 2021.
- “Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171) (Text with EEA relevance)”. European Commission, Jan 2022.
- “Joint FAO/WHO Expert Committee on Food Additives risk assessment of titanium dioxide risk released – background information”. WHO, Nov 2023.
- “Use Of Titanium Dioxide as Excipient in Human – Medicines Industry Feedback to QWP Experts/EMA Questions”. EMA, Jul 2021.

