To Issue 176
Citation: Rahmnan O, Loughran C, Senior R, “On-Body Injectors: A Promising Solution to the UK’s Systemic Anti-Cancer Therapy Crisis”. ONdrugDelivery, Issue 176 (Sep 2025), pp 42–46.
Omar Rahman, Catherine Loughran and Rachel Senior consider how on-body injectors, such as Enable’s enFuse, can help to reduce the administrative burden on the healthcare system as well as giving patients more autonomy and dignity in managing their disease.
The UK’s systemic anti-cancer therapy (SACT) infrastructure is under immense pressure. This crisis, well documented in national press and peer-reviewed literature, centres on a collision of increasing patient volume, complexity of treatments and use of maintenance therapies over fixed-duration regimens, resulting in demand exceeding capacity. Despite ongoing innovations in oncology drug development, the ability of the health system to deliver these therapies safely and efficiently is under significant strain. Patients are experiencing increasing delays, and these interruptions are not merely operational hurdles, they represent missed therapeutic windows, deteriorating prognosis and rising patient anxiety.1
“NURSES NOW FIND THEMSELVES RESPONSIBLE FOR PREPARING AND ADMINISTERING COMPLEX LVSC THERAPIES – OFTEN IN SUBOPTIMAL CONDITIONS.”
Historically, drug preparation for monoclonal antibodies resided within the aseptic environments of pharmacies. However, many UK NHS cancer centres have shifted this responsibility onto nursing staff to increase preparation capacity for cytotoxic treatments in pharmacies. Nurses now find themselves responsible for preparing and administering complex large-volume subcutaneous (LVSC) therapies – often in suboptimal conditions. The result is a rise in occupational hazards.
Data from the British Journal of Nursing show that transitioning drug handling tasks to the ward increases risks such as accidental exposure to hazardous medications and needlestick injuries.2 While well-intentioned as a solution to capacity challenges, this approach has added pressure to an already overburdened workforce. Home administration of SACT is increasingly available in the UK, both within the NHS and via private providers. Initially expanded in response to the covid-19 pandemic, it has since become a standard strategy to ease pressure on hospital services, enhance the patient experience and reduce unnecessary hospital visits.
Nurses face physical strain from manual administration of viscous, large-volume therapies via traditional syringes. Delivering LVSC injections often involves prolonged application of force while maintaining awkward postures – factors closely associated with repetitive strain injuries. These injuries, while sometimes dismissed as minor, are among the leading causes of absenteeism and attrition in the nursing profession. In busy oncology units, even minor delays in care caused by injury-related absences can ripple through patient scheduling, creating further backlogs. The prevalence of injection-related musculoskeletal disorders underscores a pressing need for ergonomic innovation in drug delivery workflows.3
Healthcare professionals (HCP) face other difficult trade-offs, too. Traditionally, many biologic therapies are warmed to near-ambient temperatures before administration, which helps to reduce injection pain, improve flow characteristics and enhance patient tolerability. However, mounting workloads and the pressure to maintain high throughput have led some HCPs to bypass warming procedures altogether on occasions. While this speeds up patient throughput, it compromises both comfort and compliance, increasing the risk of negative patient experiences and potentially undermining adherence.
Meanwhile, patients face persistent challenges in their experiences of treatment. Among the most underappreciated yet influential of these is needle anxiety. The visibility, size and invasiveness of standard syringe-needle technology contribute to pain, fear and psychological discomfort, especially in oncology, where long-term treatment regimens can involve dozens of injections. According to Alsbrooks et al, visible needles are a key driver of treatment-related anxiety, with needle phobia cited as a leading barrier to adherence in patients receiving injectable biologics. This challenge goes beyond psychological discomfort, however. Studies have shown that anxiety related to injections is associated with increased pain perception and lower patient satisfaction, even when the effectiveness of the treatment is unchanged.4
In this environment, Enable Injections’ enFuse® on-body injector (OBI) offers a unique opportunity to alleviate pressure across the oncology care continuum. The enFuse device is a wearable, hands-free OBI that delivers large drug volumes subcutaneously at a controlled, patient-responsive rate. It is designed to minimise complexity, reduce discomfort and decentralise care, addressing key barriers across patients, nurses and pharmacists amid the ongoing SACT crisis (Figure 1).
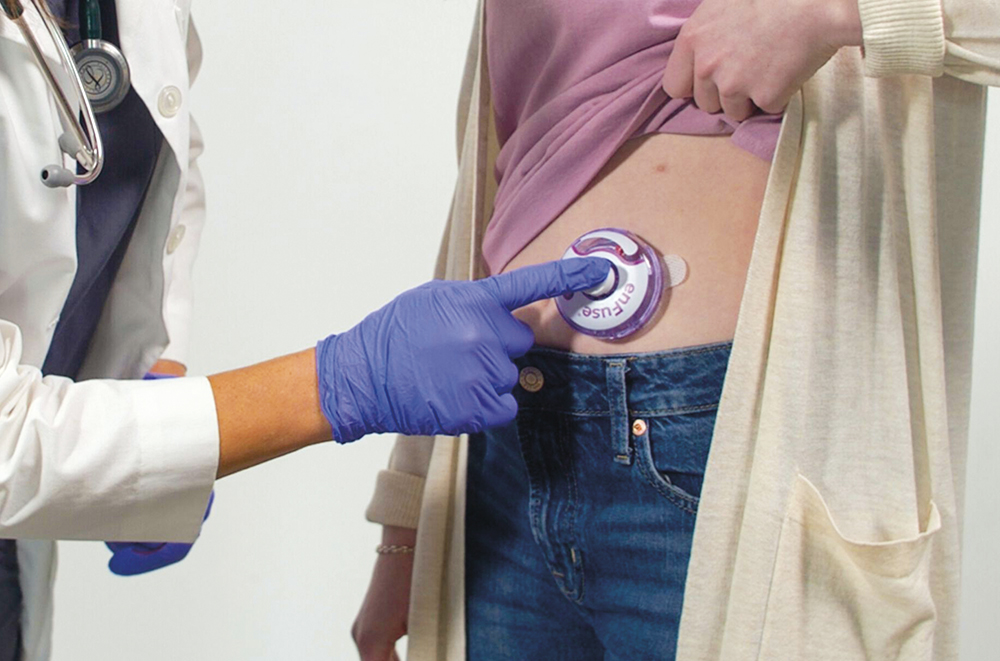
Figure 1: Starting administration with the push of a button, the enFuse OBI is designed to minimise complexity, reduce discomfort and decentralise care, addressing key barriers across patients, nurses and pharmacists.
“FOR NURSES, THE ENFUSE OBI MEANINGFULLY REDUCES THE PROCEDURAL BURDEN OF ADMINISTRATION. UNLIKE TRADITIONAL MANUAL INJECTION METHODS (E.G. SYRINGE AND NEEDLE), ENFUSE DOES NOT REQUIRE CONSISTENT MANUAL EFFORT OR FORCE TO DELIVER THE DRUG AND INVOLVES SIGNIFICANTLY FEWER STEPS.”
For nurses, the enFuse OBI meaningfully reduces the procedural burden of administration. Unlike traditional manual injection methods (e.g. syringe and needle), enFuse does not require consistent manual effort or force to deliver the drug and involves significantly fewer steps. This ergonomic simplicity limits the strain associated with repeated administration of viscous drugs and may help to reduce the incidence of injection-related repetitive strain injuries. Importantly, clinical data support this. In the IZALCO crossover study, the majority (78.9%) of HCPs preferred using the OBI for subcutaneous (SC) delivery of isatuximab. Notably, none preferred manual injection and 21.1% had no preference for either modality (OBI or manual syringe). All respondents agreed that the device was easy to use and reliable – important aspects in environments where clinical efficiency and safety are paramount.5 In addition to clinical data, two separate surveys showed a strong nurse preference for the enFuse OBI over traditional syringe and needle methods for LVSC administration, with preference rates of 97.8% and 90%.6,7
While the enFuse system is not labelled for reducing drug warming time, its integrated design offers meaningful risk mitigation in scenarios where warming is not being performed out of necessity due to ongoing systemic pressures. The system’s automatic filling and inherent ability to warm fluid during preparation and administration supports efficient workflows without compromising protocol adherence. A recent study of the enFuse system’s rapid warming capabilities highlighted that near-ambient temperature delivery can reduce patient discomfort, improve flow rates and shorten injection times. In practice, the enFuse system offers a pragmatic balance of efficiency, patient experience and regulatory alignment in high-pressure care environments.8
For patients, the benefits are particularly noteworthy. The enFuse injector uses a small, 30-gauge needle, significantly thinner than the 23- to 25-gauge needles typically used for other LVSC drugs, and it remains out of sight throughout the injection process, helping to reduce anxiety and discomfort. The device automatically adjusts delivery speed based on tissue backpressure, promoting a smoother and more comfortable injection experience. These features are not merely cosmetic, as they translate to measurable improvements in tolerability and satisfaction.
In the IZALCO trial patients received both manual and OBI delivery of isatuximab subcutaneously and were asked to express a preference after cycle six. A remarkable 74.5% of patients preferred the OBI over manual injection, while 17% preferred manual injection and 8.5% had no preference.5 These findings resonate beyond oncology. In the Wasserman et al, randomised crossover study in patients with primary immunodeficiency, all surveyed participants preferred a wearable OBI over an SC pump for immunoglobulin therapy, citing ease of use, greater mobility during infusion, less time for device set-up and less pain at the injection site.9 These results highlight the universal appeal of patient-centric delivery technologies such as enFuse.
The enFuse OBI also exhibits a strong safety performance. In the IRAKLIA study, the incidence of injection site reactions(ISRs) was significantly lower – nearly 17-fold – compared with intravenous (IV) administration.5 This has been attributed to both the smaller gauge of the needle and the adaptive pressure-based delivery system. As an example, the incidence of infusion-related reactions with daratumumab delivered subcutaneously or intravenously was 13% and 35% respectively, representing a threefold difference between the two routes of administration.10 This striking contrast suggests that the mode of delivery, given the similar mechanism of action, may meaningfully contribute to the improved tolerability profile observed with enFuse.
In the IZALCO trial, ISR rates were similarly low between manual injection and enFuse – under 1% in both groups – and all reactions were mild and self-limiting.5 These outcomes suggest that wearable OBI technology offers safety benefits without compromising reliability or dosing fidelity.
“BY TRANSITIONING APPROPRIATE PATIENTS TO AT-HOME TREATMENT SUPPORTED BY REMOTE MONITORING, HEALTHCARE SYSTEMS CAN FREE UP IN-CLINIC RESOURCES WHILE MAINTAINING THERAPEUTIC STANDARDS.”
Another compelling advantage of the enFuse OBI is its potential to support treatment outside traditional hospital settings. The device is fully wearable and intuitive, allowing patients to receive treatment in or out of hospital treatment locations with minimal supervision. This decentralisation of care holds significant promise for relieving bottlenecks in infusion clinics and SACT centres. By transitioning appropriate patients to at-home treatment supported by remote monitoring, healthcare systems can free up in-clinic resources while maintaining therapeutic standards. This strategy aligns with broader NHS initiatives to decentralise chronic care management and represents a scalable solution to SACT congestion.
Importantly, this shift does not just help hospitals, it also empowers patients. For many, treatment is not only physically taxing but logistically disruptive, involving travel, time off work or dependence on caregivers. OBI technology such as enFuse gives patients more autonomy and dignity in how they manage their disease. In diseases where long-term treatment is required, such as haematological malignancies or autoimmune disorders, the value of home administration cannot be overstated (Figure 2).

Figure 2: The enFuse OBI allows patients to receive treatment in or out of a hospital setting, using a small 30-gauge needle that remains out of sight throughout the injection process.
The enFuse OBI, therefore, addresses pain points across the entire delivery chain. It improves patient experience, reduces physical strain on nurses and supports pharmacists in maintaining best practices without sacrificing workflow. It also enables flexible site-of-care options, helping the system adapt to changing patient volumes and evolving expectations.
The SACT crisis in the UK has made clear that current infrastructure alone cannot scale fast enough to meet rising demand. What is needed is innovation, particularly innovation that supports the workforce, protects quality and empowers patients. Enable Injections’ enFuse OBI offers a compelling example of how targeted drug delivery technology can serve not just as a convenience, but as a systemic remedy. Its ability to integrate into clinical workflows, improve tolerability and shift care outside the hospital makes it more than a novel device – it becomes a practical component of care transformation.
While no single technology can resolve the entire SACT crisis, OBI systems such as enFuse are poised to play an essential role in building a more resilient, patient-centred model of oncology care. The future of SACT lies not only in the drugs we discover but in how we deliver them.
The enFuse on-body injector (OBI) and syringe transfer (ST) system were recently registered in the UK with the United Kingdom’s Medicines and Healthcare products Regulatory Agency (UK MHRA) and received European Union Medical Device Regulation (EU MDR) CE Mark approval for in-clinic use earlier this year. The enFuse OBI and ST have also received combination product US FDA approval with a specific drug for at-home self-administration, as well as approval for the enFuse System (syringe and vial transfer) from the Brazilian Health Regulatory Agency (Agencia Nacional de Vigilancia Sanitaria or ANVISA) for a range of drug viscosities and in-clinic or at-home use.
REFERENCES
- Lipanoivc D, “Routine delays: the cancer services struggling to treat patients on time”. The Pharmaceutical Journal, Dec 2023.
- Boodhoo A, “Implementing a closed system drug transfer device to enable nurses to prepare monoclonal antibody treatment”. Br J Nurs, 2024, Vol 33(10), pp S4–S8.
- Thinkhamrop W et al, “Burden of musculoskeletal disorders among registered nurses: evidence from the Thai nurse cohort study”. BMC Nurs, 2017, Vol 6(68), art 68.
- Alsbrooks K, Hoerauf K, “Prevalence, causes, impacts, and management of needle phobia: An international survey of a general adult population”. PLoS One, 2022, Vol 17(11), art e0276814.
- “ASCO: new Sarclisa data support subcutaneous administration with on-body injector”. Press Release, Sanofi, Jun 2025.
- Sánchez Avello N et al, “Subcutaneous administration of isatuximab in patients with multiple myeloma by an on-body delivery system: results of a nurse survey”. Front Oncol, 2025, Vol 18(15), art 1547108.
- Desai M et al, “Evaluating nurse preferences for a novel on-body delivery system vs. manual syringes for large-volume subcutaneous drug administration: a survey study”. Drug Deliv, 2025, Vol 32(1), art 2484278.
- Gunnerson K et al, “Enabling Rapid Drug Warming to Near Ambient”. Blood, 2024, Vol 144 (Suppl 1), pp 7550–7551.
- Wasserman RL et al, “Systemic IgG exposure and safety in patients with primary immunodeficiency: a randomized crossover study comparing a novel investigational wearable infusor versus the Crono pump”. Immunotherapy, Vol 14(16), pp 1314–1328.
- Usmani SZ et al, “Final analysis of the phase III non-inferiority COLUMBA study of subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma”. Haematologica, 2022, Vol 107(10), pp 2408–2417.

