Citation: Earl M, “Aidaptus®: Owen Mumford Introduces its Next-Generation Platform Autoinjector”. ONdrugDelivery, Issue 125 (Oct 2021), pp 22–24.
Michael Earl introduces the next-generation Aidaptus® autoinjector, explaining how this latest platform device from Owen Mumford can contribute to a faster speed-to-market, increased flexibility during drug development and improved patient outcomes.
Autoinjectors have been used as a convenient and effective means of delivering subcutaneous injections since the 1980s, and recent healthcare trends have led to an increase in their use. Autoinjectors allow patients to administer their own medication outside of acute care facilities, without the need of a healthcare professional. This helps to address a number of healthcare concerns, including an ageing population, the increase in chronic conditions among patients and pressure on healthcare systems and budgets.
A further driver of autoinjector use is the emergence, and subsequent increase, of biologics and biosimilars over the past years. More low volume formulations are being developed for these drugs to allow administration via the subcutaneous route, facilitating patient self-administration. The ensuing increase in demand for autoinjectors has created greater competition in this area, with simplicity and ease of use for the patient being a major differentiator between devices.
THE PLATFORM APPROACH
Speed-to-market is a key consideration in the drug development process, meaning that commissioning custom devices for specific drugs may not always be the most cost-effective or efficient solution. In more recent years, pharmaceutical companies have tended to opt for platform devices, which are a quicker and more flexible solution. The base platform device is designed with a wide envelope of possible delivery capabilities so that it can be used for a variety of formulations, reducing the level of testing required at the device selection stage.
However, the challenge with platform devices is that they are not designed with a specific user group in mind; the devices must therefore accommodate the needs of multiple potential patient groups.
This broad accommodation is critical to reassure pharmaceutical businesses that any use-related risk factors have been identified and addressed regardless of the patient demographics. Device developers must therefore devise inclusive testing strategies that cover patients with different levels of physical and cognitive abilities.1
BRINGING AIDAPTUS TO MARKET
A pioneer in the development and manufacture of autoinjectors, Owen Mumford Pharmaceutical Services has launched a new platform autoinjector offering next-generation benefits of flexibility and versatility (Figure 1). 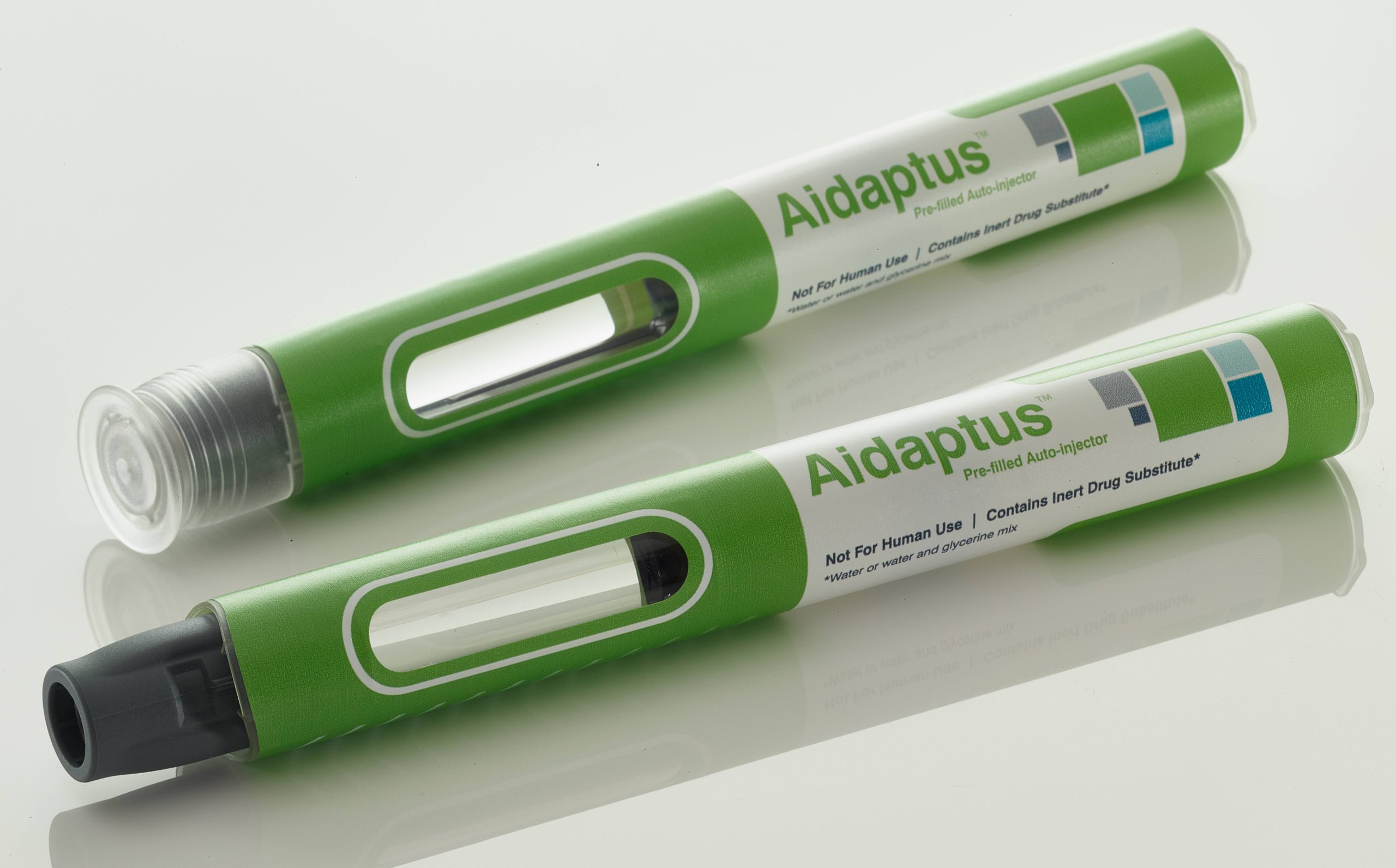
Figure 1: Aidaptus – Owen Mumford’s next-generation autoinjector.
As with all other platform devices in the Owen Mumford Pharmaceutical Services range, the Aidaptus® disposable autoinjector was designed with a focus on ease of use and patient comfort.
However, the covid-19 pandemic began during the latter stages of the Aidaptus’ development, meaning that final human factors testing could not proceed as usual. It is only through first-hand user feedback on a range of device prototypes that usability and human factors issues can be properly understood, so, to be able to move forward, the human factors team decided to prepare a new study design that would comply with the restrictions in place.

Figure 2: Aidaptus provides both audible and visual feedback to give patients confidence in their self-injection.
For example, the prototypes were all packed at least three days in advance of the study by engineers wearing PPE, and were kept sealed until needed. Rather than handing out each prototype as usual, the moderator instructed participants on which prototypes were needed at each stage and which actions they needed to take.2 This carefully adapted study provided the key data to allow the team to meet human factors requirements and to launch the product this autumn.
DESIGN BENEFITS FOR PATIENTS
Understanding the stages of needle insertion and medication delivery is key to helping patients to manage their injection process successfully and to be confident that they have administered their drug correctly. For this reason, the Aidaptus autoinjector provides patients with audible and visual notifications during the injection procedure (Figure 2).
There are audible clicks at the start and end of dose delivery, as well as a bright yellow plunger rod clearly visible through the viewing window to confirm end of dose. The window is large enough for patients to easily see the drug before administration, and to check the drug’s clarity and colour. There are no other internal mechanisms visible through the window, thereby providing an unobstructed view, as well as confidence, to the user. The needle itself is hidden before, during and after use to prevent needle exposure – a feature that may be particularly beneficial for patients with needle phobia. On completion of the injection, the safety shroud locks in place to ensure that the needle cannot be exposed and present a risk to the user before disposal.
“Needle insertion and dose delivery take place as two separate phases, with each action controlled independently. This means that the delivery of the medication does not take place until the needle is fully inserted into the patient’s skin, helping to prevent drug spillage prior to injection.”
HELPING TO PREVENT WET INJECTIONS
An issue occasionally experienced while using autoinjectors is the occurrence of “wet injections” – patients may remove the autoinjector from the injection site too early in the drug delivery process, resulting in drug spillage and wastage. Aidaptus’s design mitigates this through automatic pressure-activated needle insertion that creates a consistent user experience for all injections. Needle insertion and dose delivery take place as two separate phases, with each action controlled independently. This means that the delivery of the medication does not take place until the needle is fully inserted into the patient’s skin, helping to prevent drug spillage prior to injection.
This dual-phase action also helps to prevent syringe breakage, which can be caused by the strong springs typically used to deploy the needle for skin insertion and the plunger for dose delivery.

Figure 3: Aidaptus makes use of a novel self-adjusting plunger to prevent breaches of container closure integrity and allow for flexible syringe fill volumes.
“For maximum flexibility, the Aidaptus autoinjector is compatible with either a 1 or 2.25 mL prefilled syringe, with a minimal number of changed parts, whilst maintaining its small, discreet size (162 by 18 mm).”
Furthermore, before the product even reaches the patient, the self-adjusting plunger helps to prevent breaches of container closure integrity by limiting the backwards movement of the stopper in the syringe barrel (Figure 3). This is critical in helping to prevent microbial ingress during transit from factory to patient.
Apart from use considerations, pharmaceutical companies also need to take account of potential formulation and/or dose changes, a typical occurrence during the development and lifecycle of injectable drugs, and how these could affect their device choice. For maximum flexibility, the Aidaptus autoinjector is compatible with either a 1 or 2.25 mL prefilled syringe, with a minimal number of changed parts, whilst maintaining its small, discreet size (162 by 18 mm).
VERSATILITY AND FLEXIBILITY
Additionally, the novel self-adjusting plunger allows the use of a range of syringe fill volume options without any changes to the device and with no change in parts. The ability to adjust the drug volume whilst maintaining the same device may have significant advantages during the clinical trial phases of development, when modifications to dosages are often required to determine optimum dosing volume and frequency for the intended patient population. From a drug filling perspective, the option to use either vented or vacuum filling provides additional flexibility and may also allow for a choice of contract filling partners.
“Aidaptus is available in two presentations, one with a traditional opaque body and another with a transparent body and over-wrap, which can be printed and customised as required.”
Aidaptus is available in two presentations, one with a traditional opaque body and another with a transparent body and over-wrap, which can be printed and customised as required. This presentation allows for branding options, as well as flexibility of window size. There is scope for further optimising the patient benefits of the product by adding, for example, innovative new label technology solutions, such as new surface finishes and textures that aid grip and handling, integrated near field communication enabling connectivity options or temperature sensor technology.
PROMOTING TREATMENT OUTCOMES
Self-administration is beneficial for overall treatment outcomes, allowing patients to take more responsibility for their treatment, which improves adherence in turn. Using autoinjectors for drug administration provides greater ease-of-use and convenience for patients compared with prefilled syringes, further helping to promote adherence. Drug formulators and device developers are developing innovative solutions to further enhance the patient experience – from creating longer-acting formulations to reduce injection frequency, to reducing needle size. Owen Mumford’s Aidaptus autoinjector is designed to provide new solutions, facilitating both device selection for pharmaceutical companies and drug administration for patients.
Owen Mumford is a major healthcare company and device manufacturer that commercialises pioneering medical products in its own brand and custom device solutions for the world’s major pharmaceutical and diagnostic companies. Owen Mumford’s goal is to enhance access to diagnostics, encourage adherence to treatment and reduce healthcare costs, making a world of difference to a world of people.
REFERENCES
- Austin F, “An Inclusive Approach to the Development of Platform Medical Devices”. ONdrugDelivery, Issue 117 (Mar 2021), pp 78–80.
- Newbery M, “How Owen Mumford Carried Out Usability Testing Under Covid-19 Restrictions”. ONdrugDelivery, Issue 120 (May 2021), pp 44–46.
Previous article
INTERVIEW WITH STEPHEN M PERRY AND NICHOLAS CICCARELLINext article
A NEW MINDSET FOR COMBINATION PRODUCT DEVELOPMENT
