To Issue 154
Citation: Smith A, Brooks C, “Perfect Timing for the Automation of OINDP Testing”. ONdrugDelivery, Issue 154 (Nov 2023), pp 42–46.
Adam Smith and Clair Brooks take a look at the benefits of automation, focusing on the actuation of metered dose inhalers and multidose nasal sprays/aerosols.
“The shaking and firing that actuation of MDIs and multidose nasal
sprays/aerosols requires is repetitive, arduous and a recognised
source of variability that can be eliminated by automation.”
Key factors shaping the orally inhaled and nasal drug product (OINDP) testing landscape make the benefits of automation increasingly attractive. The reformulation of metered dose inhalers (MDIs) triggered by the Kigali Amendment to the Montreal Protocol and an increase in the application of nasal drug delivery are already adding substantially to the OINDP test burden and will continue to do so over the coming decade.
Automation reduces variability, increases productivity and simplifies training in both delivered dose uniformity (DDU) testing and aerodynamic particle size distribution (APSD) measurement.1 For a growing, increasingly geographically diverse industry facing exciting but challenging times, these are all major gains.
The shaking and firing that actuation of MDIs and multidose nasal sprays/aerosols requires is repetitive, arduous and a recognised source of variability that can be eliminated by automation. The newly launched Vertus® III+ from Copley Scientific fully automates shake, fire and shot weight measurement in a single benchtop system, while its partner DecaVertus® III, which employs the same shake and fire conditions, enables efficient firing to waste. Together, they mitigate variability and deliver a much sought-after productivity boost.
THE CASE FOR AUTOMATION
Inhaled drug delivery was first commercially realised by experts working in just a few large pharmaceutical companies, primarily in the US and Europe. Fast forward almost seven decades and the OINDP community has diversified enormously. Today, newer generic companies, innovators, and specialised contract development and manufacturing organisations flourish alongside the original pioneers in countries across the globe. An ever-growing number of people are involved in routine OINDP testing.
“Increased interest in intranasal delivery for vaccines, systemic drug delivery and nose-to-brain access is also responsible for a shift in testing patterns.”
Such proliferation is not without its challenges. The test methods applied in the development of OINDPs and for quality control (QC) are complicated by the “combination product” classification, with both device and formulation contributing to drug delivery performance. Patient practice and physiology are also influential. Automation is an obvious strategy for minimising the substantial scope for variability – from operator to operator and site to site – that this complexity brings while, at the same time, simplifying the training needed for the analyst workforce. Easier method transfer and greater productivity are also vital gains.
These benefits are especially appealing as the industry steps up to some distinct challenges. The reformulation of MDIs with propellants of lower global warming potential than the ubiquitously used HFA 134a and HFA 227a is a pressing task. Both innovators and generic companies are reliant on test data to learn how to achieve, and demonstrate, bioequivalence (BE) with new formulation chemistry. Favoured candidates for replacement are already in place – HFA 152a and HFO1234ze – but timescales are short.
Increased interest in intranasal delivery for vaccines, systemic drug delivery and nose-to-brain access is also responsible for a shift in testing patterns. These trends are encouraging more players into the OINDP market, for both new product development and, notably, drug repurposing. Taking an already approved drug and repurposing it for intranasal delivery can produce an enhanced therapeutic effect, improve ease of use/patient adherence and, at the same time, reinvigorate revenue streams via an abbreviated approval pathway. US FDA approvals for the first nalmefene hydrochloride nasal spray (May 2023) and the first over-the-counter naloxone nasal spray (March 2023) exemplify recent activity in this area.2
Against this backdrop, the case for automation is strengthening – to boost analytical productivity but also to enhance the precision of test methods by improving repeatability relative to manual methods. More repeatable and reliable data make it easier to identify and quantify differences in performance, enabling users to rigorously demonstrate BE, for example, or to learn how to robustly target nasal sprays within the nasal cavity for optimal drug delivery.
FOCUSING ON ACTUATION
US Pharmacopoeia (USP) Chapter <601> recommends “the use of a mechanical means of actuating the metering system or pump assembly to deliver doses for collection” when testing nasal sprays and aerosols, drawing attention to the potential for variability arising from device actuation3 and, while there is no such specification for MDIs, there is considerable advice to consider. For example, Bonam et al highlight storage conditions and orientation, firing rate and inhaler handling as potential sources of variability for cascade impactor testing.4
“The case for automation is strengthening – to boost analytical productivity but also to enhance the precision of test
methods by improving repeatability relative to manual methods.”
The European Pharmacopoeia (Ph Eur) highlights the need to wait for five seconds between actuations of an MDI, nasal aerosol or pressurised metered dose nasal spray to “prevent excessive cooling”.5 USP <601> similarly specifies to “wait for 5s” between actuations when a dose consists of more than one actuation and to “not cause canister cooling” when firing to waste.
An understanding of device operation is helpful when examining the impact of actuation parameters. With a nasal spray, the patient applies mechanical force to operate a metering pump, thereby squeezing formulation though an orifice within the device to release a finely dispersed dose. In contrast, with nasal aerosols and MDIs, pressing down on a cannister compresses the metering valve stem, releasing the pre-metered dose via the rapid expansion and vaporisation of a propellant. Device handling and actuation parameters are important for the following reasons:
- Shaking technique – the speed, angle and duration of shaking – determines the homogeneity of the formulation, the extent to which settled solids are re-suspended, for example, and the scale of intimate contact between the propellant and other formulation ingredients.
- Time delays between shaking and firing define the opportunity for dose separation via creaming (emulsions) or settling (suspensions), both of which can occur rapidly with some formulations.
- Applied actuation force-time profiles can impact device operation and, by extension, dose release and dispersion.
- The length of time between repeat firings can affect refilling of the metering valve chamber – priming – an essential precursor to effective delivery, as well as the degree of chilling with propellant-driven devices.
- Storage conditions, notably temperature and orientation, have been securely associated with variability in dose delivery and propensity to loss of prime, the drainage of formulation from a previously filled metering chamber.6,7
“The Vertus range comprehensively and efficiently meets requirements for automated shake, fire and flow control for MDIs and nasal sprays.”
Automated shake and fire systems eliminate this potential for variability but must be specified to handle two distinct tasks: firing to dose collection and firing to waste. The first involves actuation into a suitable dose uniformity sampling apparatus (DUSA) or cascade impactor under representative flow control, following an initial priming step. The second is shaped by specific test requirements. For example, the Ph Eur specification for dose uniformity testing of MDIs involves device priming, followed by the measurement of 10 samples in total, from the start, middle and end of label claim, with the regulatory expectation that intervening doses are fired to waste under comparable conditions. Both applications can be readily handled with a single-station shake, fire and shot weight system.
However, with some tests, the fire to waste requirement is far more substantial, notably the USP specification for dose uniformity over the entire unit life, for MDIs and nasal sprays, which involves sampling at least 10 units at the beginning and end of label claim.3 This means just 20 determinations but close to 1,000 shots to waste for a 100-dose device. These figures draw attention to the enormous burden of manual testing and demonstrate the requirement for multi-station systems.
INTRODUCING THE VERTUS® FAMILY
The Vertus range answers directly to these requirements for single- and multi-station automated shake, fire, flow control and shot weight measurement platforms. Designed to complement one another, each system applies the same rotational shaking motion to appropriately represent patient/analyst practice and, at the same time, enable straightforward method transfer. Each benefits from electronic records/electronic signatures (ERES) functionality, providing a basis for obtaining ERES regulatory approval compliant with 21 CFR Part 11 requirements.

Figure 1: Vertus III+ is a single-station automated shake, fire and flow control platform with integrated shot weight measurement for MDI and nasal drug product testing.
Vertus III is Copley’s new single-station automated shake, fire and flow control platform. Vertus III+ (Figure 1) additionally offers integral shot weight measurement, making it the flagship single-station solution. For multidose nasal solution sprays, compendial methods allow delivered mass uniformity measurement, as an alternative to delivered dose uniformity, on the assumption of formulation uniformity, making integrated shot weight measurement a valuable feature for accelerated testing.3,5 In a study with a multidose nasal spray, shot weight measurement for each of 200 doses was measured in just over 100 minutes, with no manual attention. This is an appealing approach that simultaneously produces highly repeatable data with estimates suggesting a reduction in relative standard deviation of around 50% compared with manual testing.8
These newly launched systems allow users to control all the actuation and test parameters highlighted above as potential sources of variability, and include the following key features:
- An extensive range of interchangeable interface plates, including options for the full range of Copley test method configurations for MDIs and nasal sprays, including spray force tester (SFT), thin layer chromatography (TLC) plate for spray pattern assessment, Alberta Idealised Throat (AIT) (see Box 1) and the Alberta Idealised Nasal Inlet (AINI).
- A new priming and waste module that integrates firing to waste into automated test methods, enabling compendial entire contents testing with minimal manual input.
- Intuitive user interface with large adjustable touchscreen for efficient operation for every user, with minimal training.
- Broader shaking angle and firing force range for representative testing of an even more comprehensive array of device types.
- Dedicated exhaust system to enable active extraction, if required, when working with high-potency drug substances and more flammable propellants, such as HFA 152a.
BOX 1: AN ARRAY OF TESTING CAPABILITIES
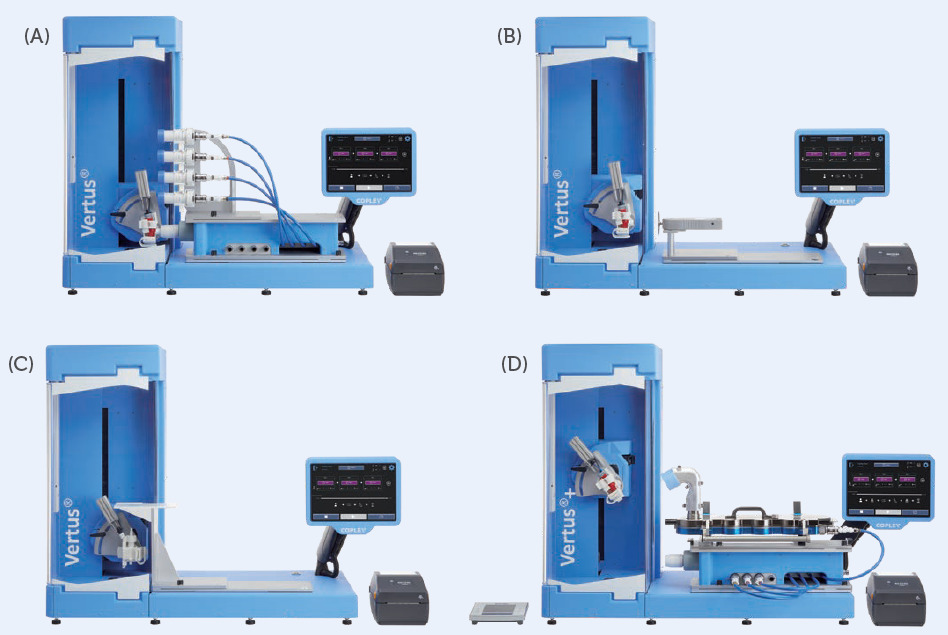
Figure 3: Interchangeable plates for Vertus III and Vertus III+ streamline all methods in the Copley testing portfolio, shown above with interface plates for (A) DUSA stack, (B) SFT, (C) TLC plate and (D) the AIT and NGI.
DecaVertus III (Figure 2) is a multistation fire-to-waste platform that applies firing force individually to each station and has dedicated air flow and waste channels for up to 10 MDIs or canisters. Its design ensures that each individual inhaler is subject to repeatable wasting under identical conditions, matched precisely to those applied by Vertus III/ Vertus III+ for simple and consistent method transfer. Dedicated waste channels are also associated with minimal cleaning and fewer blockages, even with high-volume products.

Figure 2: DecaVertus III is a multi-station automated shake, fire and flow control platform for MDIs with dedicated air flow and waste channels for up to 10 devices.
The Vertus range comprehensively and efficiently meets requirements for automated shake, fire and flow control for MDIs and nasal sprays. Studies show that automating actuation improves precision and the associated time savings can be in the region of 30% relative to purely manual analysis, notwithstanding the additional savings in training time.1,8 These are crucial gains for anyone working with MDIs and/or multidose nasal sprays/aerosols but especially for those facing the challenge of MDI reformulation or getting to grips with nasal drug product testing. In the face of challenges such as these, the case for automation has never been stronger.
REFERENCES
- Sule A et al, “Comparative evaluation of automation shake and fire system vs. manual inhaler for pMDI inhaler”. RDD 2016.
- “Information about Naloxone and Nalmefene”. US FDA, Jul 2023.
- “Aerosols, Nasal Sprays, Metered Dose Inhalers and Dry Powder Inhalers”. USP <601>.
- Bonam M et al, “Minimizing Variability of Cascade Impaction Measurements in Inhalers and Nebulizers”. AAPS PharmSciTech, 2008, Vol 9(2), pp 404–413.
- “Nasal Preparations, Nasalia”. Ph Eur 10.8.
- Schultz RK, “Drug delivery characteristics of metered dose inhalers”. J Allergy Clin Immunol, 1995, Vol 9(2), pp 284–287.
- Lewis DA et al, “Exploring the impact of formulation and temperature shock on metered dose inhaler priming”. Aerosol Sci Technol, 2016, Vol 51(1), pp 84–90.
- Smith A, Copley M, “Developing Methods for Automated Delivered Dose Uniformity Testing for Nasal Sprays”. Proc Drug Delivery to the Lungs (DDL) Conference, 2022, Vol 33.
- “Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents”. EMA, Jan 2009.
- Copley M et al, “Evaluating the Alberta Throat: An innovation to support the acquisition of more clinically applicable aerosol aerodynamic particle size distribution (APSD) data in oral inhaled product (OIP) development”. Inhalation Magazine, 2011.
Previous article
SPRAYTEC LASER DIFFRACTION: ROBUST, REPRODUCIBLE DROPLET SIZE DATANext article
UNLOCKING THE POTENTIAL OF INTRANASAL DRUG DELIVERY
