Citation: Sakuma Y, Suzuki T, “Photostability Tests of Antibody Drugs”. ONdrugDelivery, Issue 125 (Oct 2021), pp 67–70.
Yoshiko Sakuma and Tomohiro Suzuki provide an overview of Mitsubishi Gas Chemical’s OXYCAPT multilayer vial and syringe, with a particular focus on the recent investigations into the interaction of OXYCAPT’s UV barrier with the photostability of antibody drugs, compared with standard cyclo-olefin polymer and Type 1 glass.
OXYCAPT™ is a multilayer plastic vial and syringe developed by Mitsubishi Gas Chemical (MGC), offering a number of advantageous qualities as a primary drug container, including:
- Excellent oxygen and ultraviolet (UV) light barrier
- Strong water vapour barrier
- Very low extractables
- High pH stability
- Low protein adsorption and aggregation
- Silicone-oil free barrel
- High transparency
- High break resistance
- Easy disposability
- Lightweight.
MGC has continuously conducted a series of studies to confirm these excellent properties. The second half of this article will focus on a recent set of photostability tests conducted on antibody drugs using OXYCAPT, Type 1 glass and cyclo-olefin polymer (COP). Before that, the first half of this article will provide an overview of OXYCAPT multilayer plastic vial and syringe (Figure 1).

Figure 1: The OXYCAPT multilayer plastic vial and syringe.
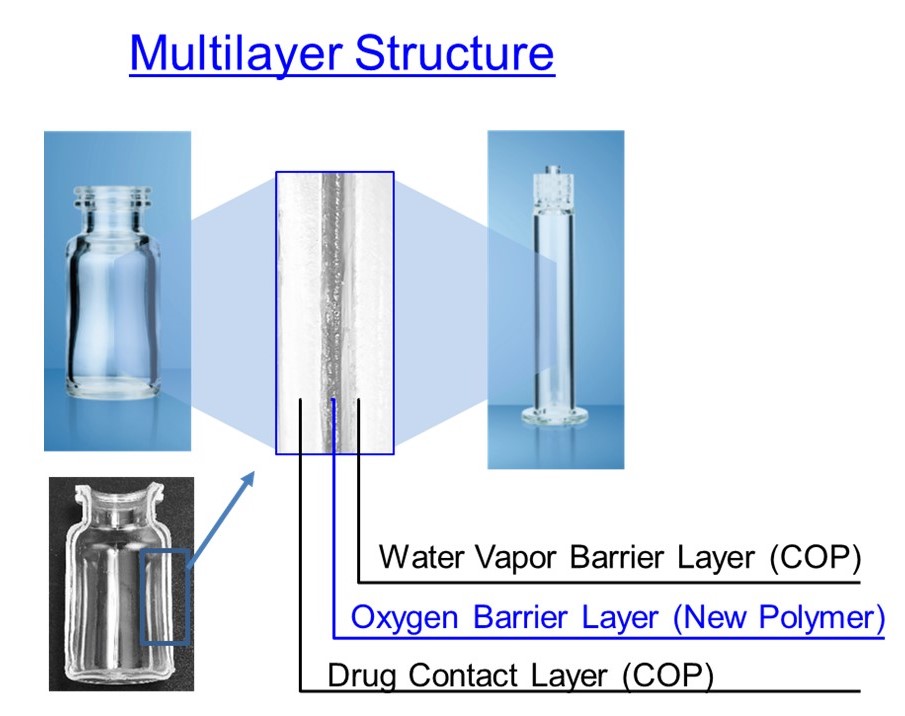
Figure 2: Multilayer structure of OXYCAPT.
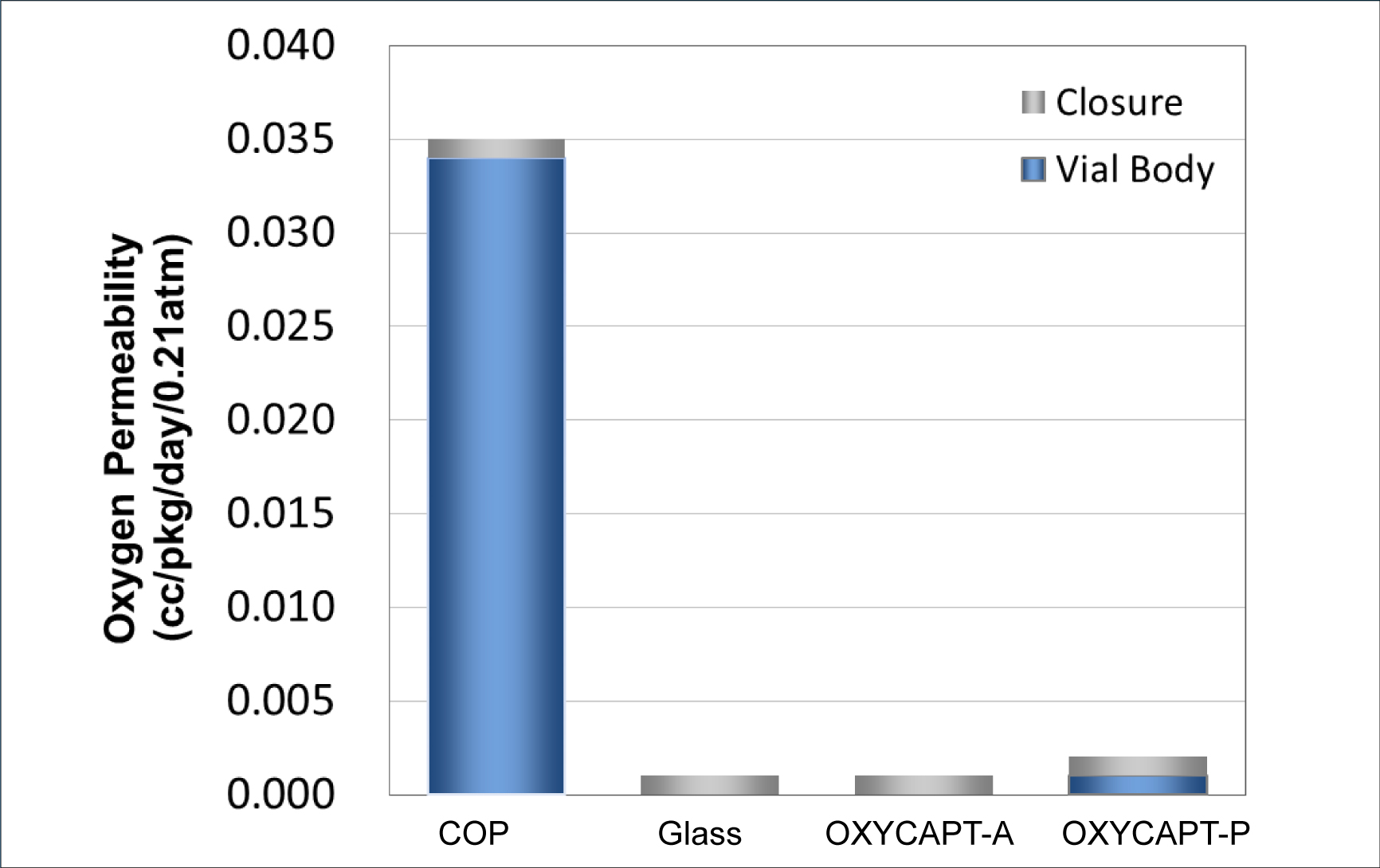
Figure 3: Oxygen permeability comparison of a typical COP, glass, OXYCAPT-A and OXYCAPT-P.
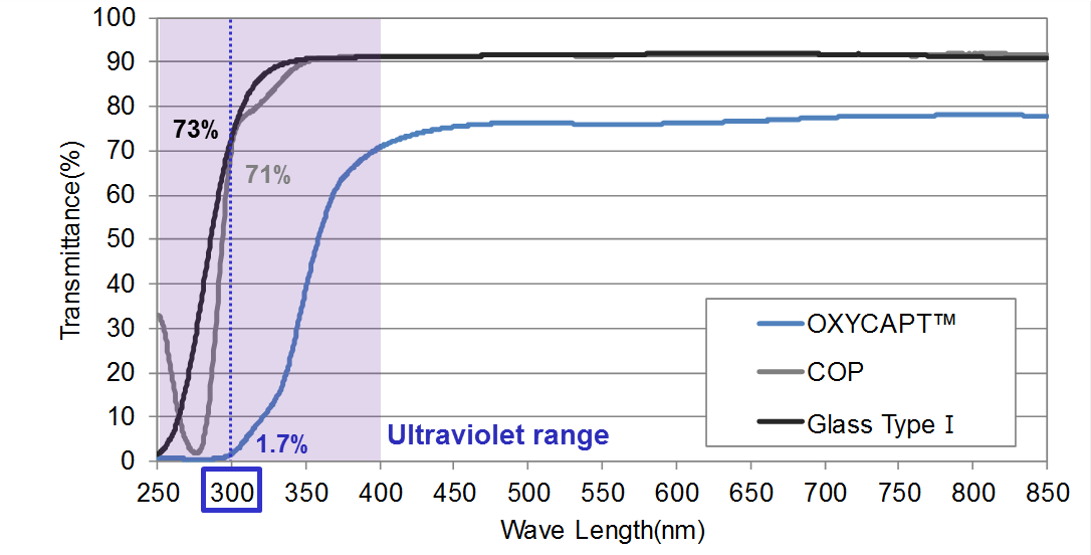
Figure 4: UV light transmittance comparison of a typical COP, Type 1 glass and OXYCAPT.
The material consists of three layers – the drug contact layer and the outer layer are made of COP, and the oxygen barrier layer is made of MGC’s novel polyester (Figure 2).
OXYCAPT OVERVIEW
There are two types of OXYCAPT multilayer plastic vial and syringe – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A offers a glass-like oxygen barrier. According to internal studies, thanks to its oxygen-absorbing function, OXYCAPT-A can maintain lower oxygen concentrations in the headspace than Type 1 glass. OXYCAPT-P also provides an excellent oxygen barrier, although there is no oxygen-absorbing function. For example, the oxygen barrier of an OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial (Figure 3).
“Although about 70% of 300 nm UV light transmits through glass and COP, only 1.7% transmits through OXYCAPT (Figure 4). MGC has confirmed that this feature contributes to the stability of biologics.”
OXYCAPT also provides an excellent UV barrier. Although about 70% of 300 nm UV light transmits through glass and COP, only 1.7% transmits through OXYCAPT (Figure 4). MGC has confirmed that this feature contributes to the stability of biologics.
While OXYCAPT cannot reach the performance of glass with respect to acting as a water vapour barrier, its properties are similar to those of COP, which has been used for injectable drugs for a long time. This means that OXYCAPT easily meets the requirements of a water vapour barrier set out by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.
Studies have shown an extremely low level of extractables from OXYCAPT. One study was conducted to confirm the levels of volatile, semi-volatile and non-volatile impurities from OXYCAPT. Water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were selected, and impurities were measured by gas chromatography mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with the control, impurities were not detected in the OXYCAPT containers. A second study confirmed that inorganic extractables levels from OXYCAPT were similar to those from COP, which is well known for being an extremely pure polymer, and with a better extractables profile than Type 1 glass. Lower levels of inorganic extractables are known to contribute to better pH stability in drug products.
OXYCAPT vial and syringe are produced by co-injection moulding technology. Although this technology has been used in the production of beverage bottles for many years, MGC is the first company to succeed in applying it to the production of multilayer plastic syringes. MGC has also developed inspection methods for testing the oxygen barrier layer. All of the containers are fully inspected by state-of-the-art inspection machinery.
MGC can offer bulk vials, ready-to-use (RTU) vials and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-based nest and tub formats. The nest and tub are mainly sterilised using gamma rays. There are 2, 6, 10 and 20 mL variants for vials, and 1 mL long and 2.25 mL variants for syringes (Table 1).
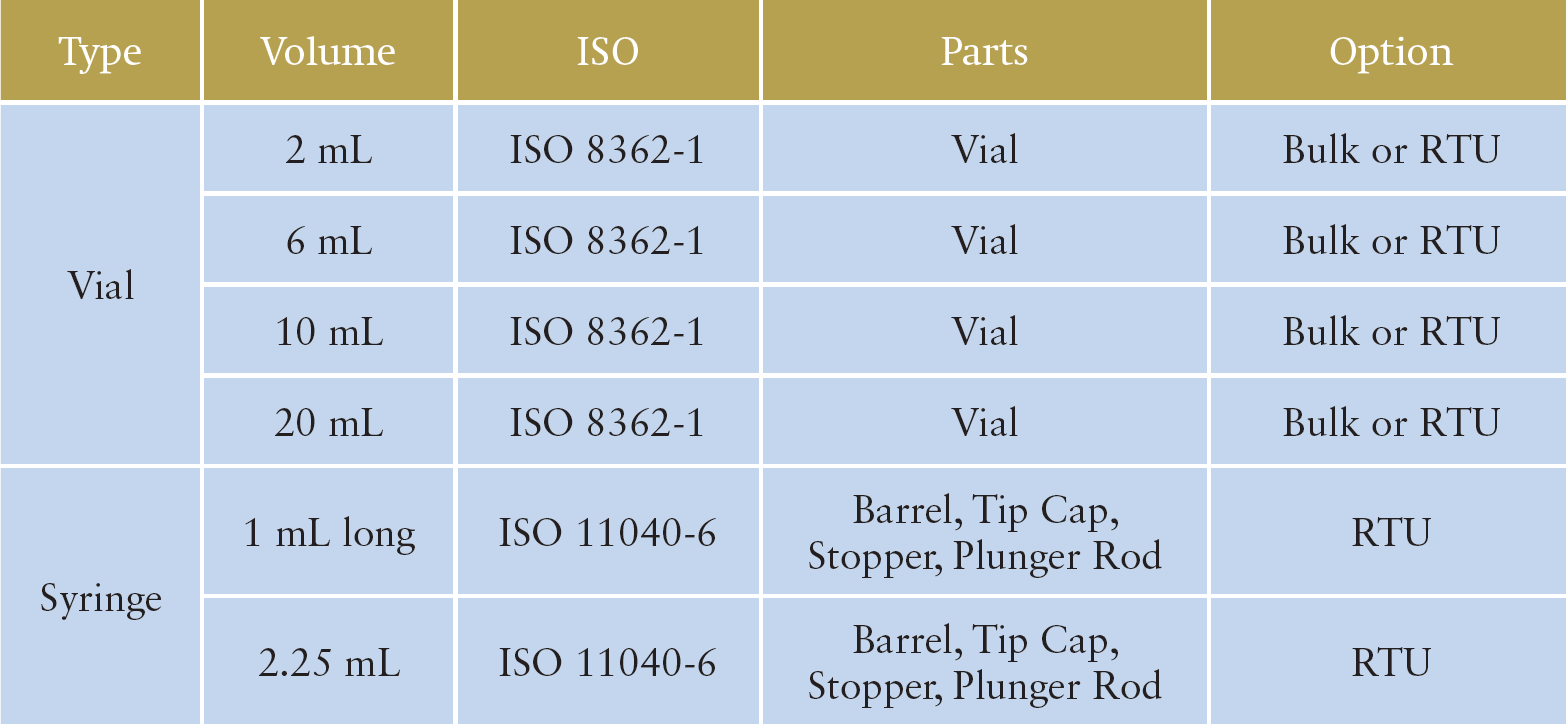
Table 1: MGC’s OXYCAPT product portfolio.
MGC is willing to provide samples for initial testing free of charge. Each polymer meets the requirements of United States Pharmacopeia (USP) regulations USP<661>, USP<87> and USP<88>, as well as those of the European Pharmacopeia, and has been filed in the US FDA’s drug master file (DMF). The vials and syringes are also compliant with each pharmacopoeia and have been filed in the DMF. The syringes are produced and controlled in accordance with ISO 13485. The primary target market for OXCAPT is the therapeutic application of biologics. As mentioned in ICH Q5C (Stability of Biotechnological/Biological Products), oxidation is one of the causes of protein instability. As such, the oxygen and UV barrier properties of OXYCAPT will definitely contribute to the stability of biologics stored within. Additionally, MGC believes that OXYCAPT would be well-suited to emergency adrenaline, which is well-known as an oxygen-sensitive drug, because OXYCAPT combines both an oxygen barrier equivalent to Type 1 glass and the breakage resistance of a polymer. Furthermore, some drug developers have recently started evaluating the OXYCAPT vials for their gene and cell therapy; the RTU vial is sterilised by gamma radiation, making it ideal for protein-based drugs.
“MGC believes OXYCAPT multilayer plastic vial and syringe with its strong oxygen and UV barrier is the best candidate for antibody drugs.”
PHOTOSTABILITY TESTS
Studies have shown that the oxidation of antibody drugs is caused by oxygen and UV light and that such factors have a negative impact on their potency and halflife in blood. Also, the methionine residue located on the CH3 domain of the fragment crystallisable (Fc) region of an antibody is structurally exposed to the environment and is likely to be oxidised. Moreover, some researchers have reported that the oxidation of said methionine tends to bring about deterioration of structural stability and aggregation upon heat treatment. Therefore, MGC believes OXYCAPT multilayer plastic vial and syringe with its strong oxygen and UV barrier is the best candidate for antibody drugs. To test this belief, MGC recently conducted photostability tests of commercially available antibody drugs in OXYCAPT, Type 1 glass and COP containers.
To obtain results in a shorter time span, MGC first removed any polysorbate-80 (PS-80), a non-ionic surfactant as a stabiliser, from commercially available antibody drugs based on immunoglobin G (IgG) subtype IgG1. Secondly, OXYCAPT-P, COP and Type 1 glass vials and syringes were filled with the antibody drugs without PS-80 in a nitrogen chamber. All the samples were exposed to light (totalling 1.2 million luxhours) at 25°C. Finally, oxidation rates and quantities of aggregates in the antibody drugs were measured.
Oxidants of methionine were quantified by liquid chromatography with tandem mass spectrometry (LC-MS-MS). For the protein aggregates, soluble proteins were analysed by size chromatography (SEC), with micro-particle counts and size distributions obtained by flow injection analysis (FIA). Throughout the study, commercially available antibody drugs that had not been exposed to light were used as a control.
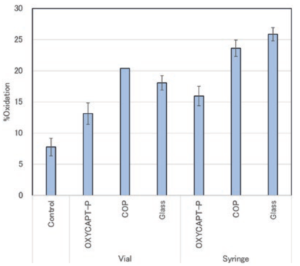
Figure 5: Oxidation rate of methionine.
With regard to the quantitative analysis of oxidants by LC-MS-MS, each average oxidation rate (+/- the standard distribution) of the samples’ DTLMISR peptide sequence, including methionine located on the CH3 domain of the Fc region, was calculated (Figure 5). The study results showed that the oxidation rate of the antibody drug contained in the OXYCAPT-P vial and syringe was lower than that of those in Type 1 glass and COP containers. Considering that the oxidation rate in Type 1 glass, which has a strong oxygen barrier, was the same as that in COP, with a poor oxygen barrier, MGC believes that the exposure to UV light accelerated the oxidation rate of the tested antibody drugs.
MGC also used SEC analysis to calculate what percentage of the total area of each peak concerned oligomers, monomers and fragments of the antibody drugs (Figure 6). Compared with Type 1 glass, the rate of oligomers and fragments in OXYCAPT-P and COP was lower, the polymer containers maintaining a higher rate of monomers. Also, a T-test showed that the OXYCAPT-P syringe maintained a significantly higher rate of monomers than the COP equivalent.
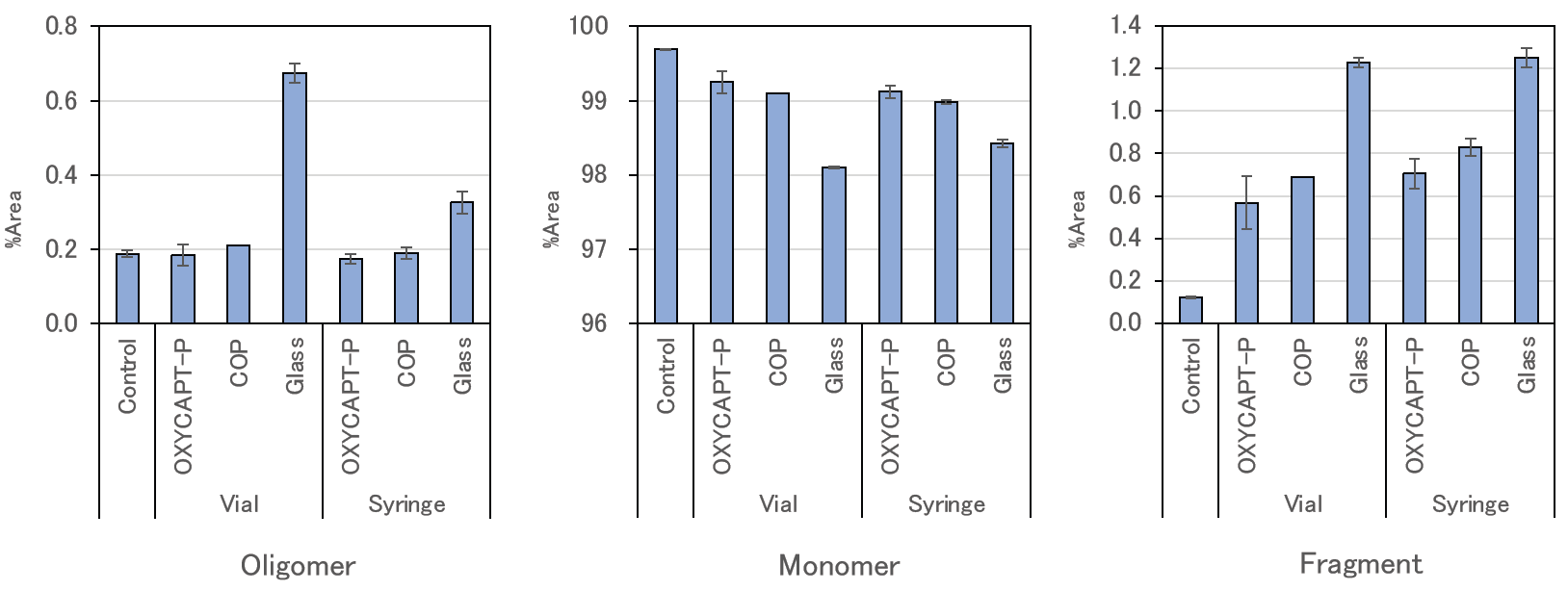
Figure 6: Results of SEC-HPLC (percentage of each ingredient).
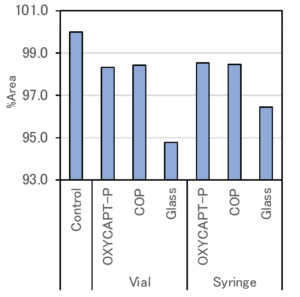
Figure 7: Average monomer rate of each container.
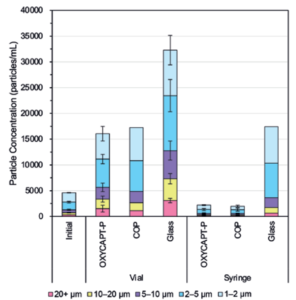
Figure 8: Particle concentrations in each container.
Figure 7 shows the average proportion of monomer in each container with the control regarded as 100%. Since a loss of monomer was observed in all the containers, it can be assumed that decompositions and aggregations of antibody drugs were caused by the exposure to UV light.
Figure 8 shows the particle concentrations in each container obtained by FIA. The particle concentrations in OXYCAPT-P and COP were much lower than those in Type 1 glass, while no significant difference was observed between COP and OXYCAPT-P. Although the vials’ particle concentrations were generally higher than the syringes’, it can be assumed that the bigger head space in the vials led to more vibration stress on the antibody drugs during transport, and, as such, reducing said head space will prove a useful measure for preventing the increase of particles.
CONCLUSION
In conclusion, the latest results have verified OXYCAPT’s superior properties. In addition to the special features of COP, such as its strong water vapour barrier, high breakage resistance, very low extractables and low protein adsorption, OXYCAPT can provide excellent an oxygen and UV barrier. MGC believes OXYCAPT brings significant and varied benefits to the rapidly growing biologics and gene/cell therapy sector.

