Citation: Bauert D, Kumar Busimi A, “Expert View: Primary Packaging for Wearable Injectors. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 26-28.
Dominique Bauert and Anil Kumar Busimi provide an overview of existing wearable injector solutions and new requirements, with a focus on the role of the primary packaging inside the devices.
Patients have used pen and autoinjectors at home to treat chronic conditions like diabetes and rheumatoid arthritis for quite some time. Home administration of novel biological drugs presents many challenges. Subcutaneous injection of viscous biologic drugs is possible if injected in high volumes or over a longer duration. To achieve this, a new class of drug delivery platform, wearable injectors, has gained wide attention in the industry.
Undergoing medical treatment is a time of intense stress for patients. In addition to the illness itself, patients sometimes endure multiple trips to doctors’ offices. Self-injection gives patients the ability to avoid these trips and maintain a sense of normality and autonomy. And it’s exactly this requirement for patient acceptability, for comfort and convenience, that has become a major priority for drug makers.
Biologics now comprise a substantial portion of the pharmaceutical industry’s development pipeline yet designing corresponding self-injection scenarios is still a challenge. The first reason lies within the drug formulation itself. Their high doses, particular storage requirements, and high viscosity mean that many biopharmaceuticals need to be administered intravenously (IV). Patients receiving such biologics have to travel to a hospital or doctor’s office for treatments. To create the necessary conditions for biologics to be self-administered, pharma companies have devised formulations that are capable of being injected subcutaneously (SC) or intramuscularly (IM). Either this happens through pen injectors or prefilled syringes, the latter often supplemented by auto injection or safety devices.
“At the heart of any wearable injection device sits the primary packaging. It is a crucial component because it is the main
point of contact between the drug and the device, yet its importance is often overlooked.”
However, SC self-administration faces some challenges when applied to biologics; again often due to their large volume, high viscosity, or both. While many biologic formulations require volumes of up to 10 mL, most pen injectors and prefilled syringes are currently designed to deliver a maximum of 2.5 mL per injection. The strategy to increase the concentration of the formulation might reduce the volume yet leads to an increase in viscosity and subsequently the requirement for a higher injection force. Either way, the injection of large volumes and viscous liquids mostly involves more discomfort for the patient. In addition, there tends to be an upper limit of the time a patient can hold an injection device in place during administration, which amounts to approximately 15 seconds.
Because of these constraints, there has been a consistent focus within the industry on the design of wearable large-volume injectors (LVI) to administer biological drugs that require longer injection times due to higher volumes and/or higher viscosity.
LARGE-VOLUME WEARABLES FOR BIOLOGICS – AN OVERVIEW
Wearable injectors adhere to the body and administer highly viscous medicines at high volume. There are already several examples on the market, including devices from West Pharmaceutical Services, Ypsomed, Enable Injections, Sonceboz, Sorrel Medical, Subcuject, Weibel CDS (all featured elsewhere in this issue).To make them more patient-centric, many of the wearable LVIs currently in development (see Figure 1) are capable of delivering volumes of 20 mL and more over scheduled intervals.

Figure 1: A selection of the companies currently developing wearable LVIs (images courtesy of the mentioned companies).
For example, Amgen’s Neulasta® Onpro® kit combines the biological medicine that gives a boost to the immune system with a wearable injector that adheres to the patient’s body (Source: Letter to Shareholders, Amgen, 2017, p 8). The device is affixed to the patient, and medicine is delivered over a period of 45 minutes, approximately 27 hours after chemotherapy treatment, allowing patients to avoid a return to the hospital.
Additionally, medical device designers can incorporate a wide array of features and components into a device. Figure 2 provides an overview of device features and design considerations in available wearables.
At the heart of any wearable injection device sits the primary packaging. It is a crucial component because it is the main point of contact between the drug and the device, yet its importance is often overlooked.
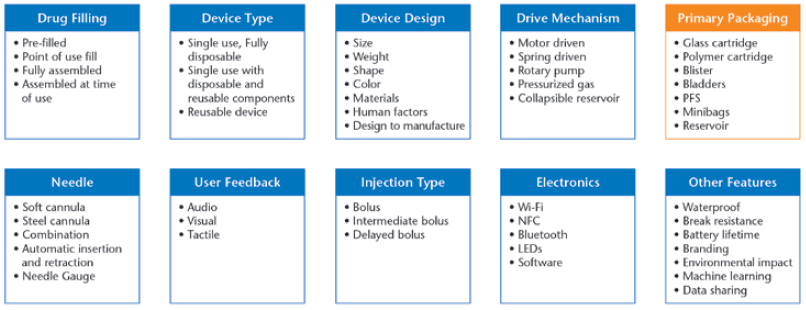
Figure 2: Overview of device features and design considerations in available wearable injectors.
PRIMARY PACKAGING – WHAT IS NEEDED?
Wearable injectors rely on multiple primary packaging systems. Some use flexible bladders or bags, others use rigid glass or polymer containers such as syringes or cartridges. Whilst some injectors are filled at the point of use, the trend in wearable LVI injectors has leaned toward prefilled doses as they reduce the risk of medication error. Irrespective of the material, each of these prefilled containers must fulfil specific requirements.
Drug Stability
The term primary packaging refers to the fact that the container comes in direct contact with the drug, including stoppers or closures, plungers, and lubricants such as silicone oil. The complex molecular structure of long-chain biologics increases risk of the drug interacting with the components of the container, which can lead to interactions and may reduce its efficacy. For drug developers, these interactions are impossible to predict, which is why extensive risk analyses and stability tests are required before regulatory approval. They can minimise the number of variables by relying on high quality containers that exhibit a reduced extractables and leachables (E&L) profile from the start. More often, such containers are made from high-quality borosilicate glass, or high tech polymers like COC or COP, feature reduced, bakedon, or cross-linked silicone, and incorporate specialised elastomer components that come with a reduced E&L risk.
Functionality
Container functionality comprises numerous aspects that again contribute to the stability of the drug. For example, container closure integrity (CCI) has become an area of concern, as appropriate test methods for verification of the sterility of polymer bags or bladders are currently not in place.
Proper function also contributes to a convenient administration – which is the ultimate goal of any wearable injector. Here, the geometrical precision of rigid containers like cartridges or syringes already supports dosage accuracy and ease of use thanks to reduced break-loose and gliding forces.
Whether glass or polymer offers the better functionality depends on the individual application. Both materials have their advantages. Borosilicate glass is still the gold standard to package biologics due to its high chemical stability and proven track record. Polymer, in turn, can offer more design freedom. New co-creation approaches enable the pharma company and the design manufacturer to adjust the container design to the device, creating ever smaller and more discreet applications, instead of building the wearable around an existing type of primary packaging.
In any case, drug makers should investigate the best possible material route together with a packaging manufacturer who offers a complete portfolio of both glass and polymer solutions.
Economics in Fill & Finish
The narrow patient population for biologics has had a downstream effect on filling operations, which naturally also effects the primary packaging for biologics to be used within a wearable device. Most biologics are produced in smaller batches, and pharma manufacturers have been working on ways to fill these small quantities cost-effectively. The industry has thus seen a trend towards ready-to-use (RTU) containers that rely on the same industry standard nest and- tub format for each container type. This allows the same filling line to be used for multiple container formats with minimised changeover time in between. In addition, RTU packaging arrives sterile at the manufacturing line, allowing for fully aseptic manufacturing.
While extensive experience with rigid RTU containers such as syringes, vials, and increasingly RTU cartridges is available in the industry, comparable small volume (<100 mL) scenarios with flexible polymer containers still lag behind.
FINALLY – IS THERE AN IDEAL PRIMARY PACKAGING FOR WEARABLES FOR BIOLOGICS?
The cartridge has emerged as the primary packaging container that fulfils the diverse requirements of wearables for biologics. With cartridges, designers have wide latitude to choose container length, volume, diameter, neck and flange design, and the stopper and plunger material. Another benefit of cartridges is that many of their functional parameters, particularly glide force, have been tested to full extent according to ISO standards. Finally, this type of primary packaging container is available in different materials, delivered in bulk and recently also as an RTU variant in industry standard tub and nest configuration.
This article draws from an earlier work of Busimi & Bauert, published in Pharmind 2019.
Previous article
DEVELOPMENT OF AN OSMOTIC LARGER VOLUME WEARABLE BOLUS INJECTORNext article
TRAINING PATIENTS IN THE USE OF WEARABLE INJECTORS
