To Issue 165
Citation: Buday J, “Prioritising Sustainability in Pharma”. ONdrugDelivery, Issue 165 (Sep/Oct 2024), pp 44–48.
Jessica Buday discusses how, in partnership with West Pharmaceutical Services, Corning’s Viridian glass vials are able to simultaneously improve the sustainability and efficiency of pharmaceutical fill-finish lines compared with traditional ISO glass vials, due to their reduced glass volume and proprietary external polymeric coating.
This article is based on an article originally published in Pharma’s Almanac in June 2024, written by Jessica Buday and Dr David Alvaro.
Historically, the pharmaceutical sector has trailed behind other industries in vigorously addressing environmental sustainability – however, the tide is turning. Acknowledgement of the need to mitigate the environmental footprint of pharmaceutical processes, from development and manufacturing to distribution, is growing. This shift is embodied by efforts such as the “Sustainable Markets Initiative”, which has united a diverse coalition of over 500 entities, including global companies and leaders, to establish and pursue ambitious sustainability benchmarks across the pharmaceutical and healthcare sector.
An inflection point occurred in 2023, when executives from prominent pharma and life sciences companies joined forces within the Sustainable Markets Initiative Health Systems Task Force. The task force penned a candid open letter1 to the industry’s suppliers, highlighting the pharmaceutical sector’s sizeable environmental impact – the industry is responsible for roughly 5% of climate change factors, with its supply chains contributing a significant portion of that impact. This collaborative call to action emphasised the urgency of setting and achieving robust sustainability goals.
Many pharmaceutical company suppliers are already on this path, ambitiously integrating sustainability criteria into their strategies. It is no longer just about the immediate utility of a product; the focus has expanded to encompass the environmental ethos of suppliers and their capability to support the client’s sustainability objectives.
In particular, attention has been directed towards reducing the impact of secondary packaging, such as cartons, labels, packaging inserts and other packing materials. More recently, the industry has begun to tackle the more complex challenge of the impact of primary packaging. This presents a more significant hurdle, owing to the stringent regulatory requirements associated with materials that are in direct contact with the drug product. Every change must be carefully assessed to ensure safety and efficacy, along with environmental footprint.
A COMPREHENSIVE SUSTAINABILITY STRATEGY
Corning’s approach to sustainability combines product innovation and practicality to achieve wider adoption without compromising quality. This includes a commitment to ambitious environmental goals – aiming for a 30% reduction in direct and indirect greenhouse gas emissions (Scope 1 and 2) and a 17.5% absolute reduction in the broader emissions (Scope 3) by 2028, using 2021 as a baseline. This comprehensive strategy extends throughout the company’s entire value chain, including the procurement of goods and services, production of capital goods and management of fuel and energy resources, as well as logistics.
In accordance with the Paris Agreement and the Science Based Targets initiative (a partnership between the Carbon Disclosure Project, United Nations Global Compact, World Resources Institute and World Wide Fund for Nature), Corning is dedicated to a sustainability ethos that spans from design and product development through to manufacturing and shipping. Recognising the finite nature of the planet’s resources and the pressing issue of climate change, the company is reimagining product development from the ground up.

Figure 1: Corning’s Viridian glass
vials present improvements in both
efficiency and sustainability, offering a
greener option without compromise.
INNOVATING SUSTAINABLE SOLUTIONS IN PHARMACEUTICAL PACKAGING
Building on the recent collaboration between Corning and West Pharmaceutical Services, which brings together more than 200 years of experience, Corning® Viridian® glass vials address performance and sustainability together in a system level containment solution (Figure 1). This exclusive supply and technology agreement provides pharmaceutical partners with access to some of the most innovative products in parenteral drug delivery. Both Corning and West have explored novel ways to support customers’ sustainability goals – a commitment evident in their growing focus on renewable energy, waste reduction and the creation of products with sustainable features, all crafted in close collaboration with customers.
Corning is committed to innovation, which fuels its ability to develop sustainability programmes and products that minimise environmental impact without sacrificing quality or performance. This includes efforts to revolutionise pharmaceutical glass packaging – looking beyond manufacturing processes to reduce emissions and expanding the search for opportunities for improvement at every stage.
“A crucial design requirement was that the vials serve as direct replacements for current Type I borosilicate glass products to ensure optimal compatibility and ease of transition for established filling processes.”
The innovative design of Corning Viridian vials took shape under the guiding principles of form, fit and function. Each aspect was engineered to meet international standards and seamlessly integrate with current drug product filling systems and the full range of pharmaceutical processing steps, such as terminal sterilisation, lyophilisation and cold chain storage conditions, including extremely low temperatures (Figure 2). A crucial design requirement was that the vials serve as direct replacements for current Type I borosilicate glass products – the standard glass vial primary packaging for most injectable drug products – to ensure optimal compatibility and ease of transition for established filling processes.

Figure 2: Viridian vials can seamlessly integrate with existing fill-finish and transport infrastructure.
While vials produced using less glass are on the market, they often suffer from performance and quality limitations. Viridian vials use a proprietary external polymeric coating technology to overcome these potential challenges. This coating, which has been used to protect other Corning pharmaceutical glass products, such as Valor® Glass, enables Corning to reduce the thickness of the vial walls without impacting their ability to withstand typical forces during fill-finish processes.
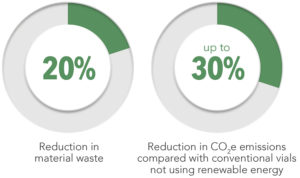
Figure 3: Viridian vials represent a 20% reduction in material waste and a 30% reduction in emissions compared with traditional vials not using renewable energy.
Although they are made with 20% less glass, enabling up to a 30% reduction in cradle-to-gate Scope 3 emissions(Figure 3), Viridian vials present a drop in solution that fits effortlessly into pharmaceutical manufacturing that uses Type l borosilicate glass vials. The innovative coating preserves the integrity of the vial, and Viridian vials have demonstrated an up to 20–50% increase in fill-finish line throughput efficiency by enabling smoother vial flow and reducing the incidence of line stoppages due to equipment jams or vial breakages (Figure 4). These efficiency benefits ultimately decrease both glass waste and production emissions. These gains are complemented by a commitment to using renewable energy in the manufacturing supply chain for Viridian vials.

Figure 4: Viridian vials can improve filling line efficiency by 20–50% compared with conventional glass vials.
CONSIDERING THE FULL VIAL LIFECYCLE
To further validate the material and emissions reduction advantages, Corning sought to authenticate the sustainability impact of Viridian vials through a third-party-verified lifecycle assessment (LCA). In this case, a “cradle-to-gate” LCA that evaluated the environmental impacts from extraction of raw materials through to finished product vial manufacturing was conducted by Sphera (Chicago, IL, US), a leading provider of environmental performance and risk management software, data and consulting services. It should be noted that this LCA did not include the downstream impacts of distribution, use or disposal at the end of life, which are additional user-dependent opportunities for emissions savings.
The LCA confirmed that the more sustainable design of Viridian vials, coupled with Corning’s manufacturing efficiency, offers significantly lower emissions for customers using these vials in their manufacturing processes compared with traditional vials. Beyond the factory walls, these improvements also offer drug manufacturers a pathway to reduce their Scope 3 emissions – those not directly produced by the company but that are associated with their operations and supply chain – which are typically challenging to quantify and manage.
Reducing the wall thickness and therefore the quantity of glass used in each Viridian vial by 20% compared with conventional vials reduces cradle-to-gate Scope 3 emissions by up to 30%. Using less glass per vial reduces the natural resources consumed in their production. Furthermore, Corning estimates the reduction of pharmaceutical glass usage by 20% may prevent nearly 30,000 tons (>27 million kg) of glass from entering the waste stream each year (Figure 5). Less material per vial also reduces the vial’s weight, reducing CO2 equivalent (CO2e) emissions related to transport, enabling more efficient use of packaging and potentially reducing shipping costs.
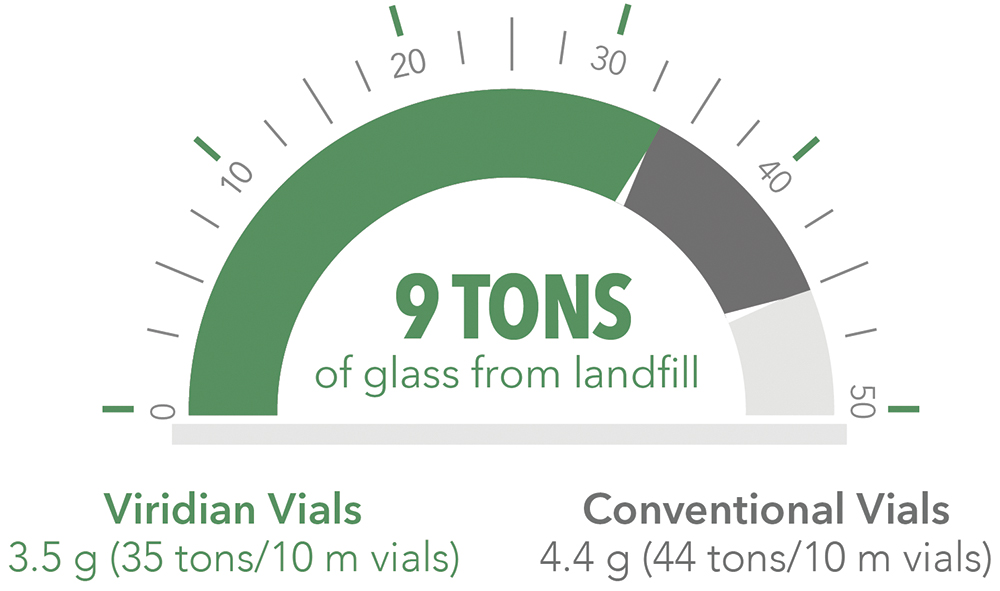
Figure 5: Using Viridian vials rather than traditional glass equivalents can reduce the amount of glass being sent to landfill by up to 9 tons for every 10 million vials produced.
A 2 mL Viridian vial weighs 3.5 g, compared with 4.4 g for a standard 2R ISO vial. Therefore, for every 10 million Viridian vials produced, up to 114,000 kg of CO2e emissions could be avoided – the amount produced by burning over 48,830 L of petrol – with the added environmental benefit of preventing 9 tons of glass from reaching landfill (Figure 5).
This material reduction, combined with using 100% renewable electricity in the manufacturing process, enables a modest reduction in customers’ overall Scope 3 emissions. Even when compared with conventional vials also made with 100% renewable electricity, the use of Viridian vials can reduce emissions by 15%.
“Viridian vials support increased filling line speed and throughput, enhancing productivity by 20–50% compared with traditional glass vials.”
SUPERIOR PERFORMANCE AND DURABILITY OF VIRIDIAN VIALS
Vial-to-vial and glass-to-metal friction, along with vial impact events, are leading sources of reduced efficiency and yield in fill-finish processes. In laboratory testing, Viridian vials have shown up to 30 times fewer glass-related defects compared with conventional borosilicate glass vials. Notably, Viridian vials support increased filling line speed and throughput, enhancing productivity by 20–50% compared with traditional glass vials.
The proprietary external coating significantly reduces the creation of glass particulates and has demonstrated an up to 96% reduction in peak particle count on commercial filling lines, which could have a significant impact on reducing drug recalls due to particulate contamination.
In a trial with Optima Pharma (Schwäbisch Hall, Germany),2 a producer of fill-finish machinery, Viridian vials were put to the test. Optima assessed the rate of tip-overs, jams and breakage among standard 2R borosilicate vials and 2 mL custom Viridian vials, running 450 vials per minute through more than 120 cycles to simulate the processing of over 200,000 vials for each group. Viridian vials demonstrated comparable or superior resistance to breakage – no breakage was observed for the Viridian vials during washing, depyrogenation, accumulation, singulation or starwheel transfer. In addition, no functional issues were observed with washing or depyrogenation. Viridian vials also exhibited a sixfold reduction in tip-over rate compared with the conventional vials. These improvements were realised without any alterations to the existing machinery, underscoring the vials’ adaptability and superior performance.
ONGOING EXPLORATION OF EXTENDED BENEFITS OF VIRIDIAN VIALS
“Since the 2023 launch of Viridian vials, early adopters in the pharmaceutical industry have already begun to see the advantages of switching to this more sustainable option and, alongside observed improvements in the filling process, further benefits continue to be uncovered.”
Since the 2023 launch of Viridian vials, early adopters in the pharmaceutical industry have already begun to see the advantages of switching to this more sustainable option and, alongside observed improvements in the filling process, further benefits continue to be uncovered. For example, the reduced wall thickness of Viridian vials can enhance heat transfer efficiency during lyophilisation, potentially reducing cycle times and increasing throughput. This efficiency can not only reduce the cost per dose but also accelerate overall production rates.
Furthermore, the reduced wall thickness allows for filling a larger volume within the same vial size, enabling a greater quantity of medication per vial, especially for multidose formulations. This could lead to fewer vials used overall, increasing sustainability, and potentially delivering cost savings and productivity gains. In commitment to environmental stewardship, Corning is actively investigating more sustainable options for the secondary packaging of Viridian vials.
MERGING HIGH PERFORMANCE WITH SUSTAINABLE DESIGN
Corning and West are committed to enacting real change, embodied by the companies’ dedication to climate initiatives, sustainable design and responsible stewardship, and are looking forward to forging partnerships within the pharmaceutical industry to expand the sustainability ethos across every link of the drug supply chain. This is where Viridian vials really deliver true value, with demonstrable benefits and careful consideration to maintaining specification and operational efficiency. However, this is just the start – the Corning–West collaboration will continue to innovate and further improve to support pharma’s sustainability goals.
Corning®, Valor®, and Viridian® are trademarks of Corning Incorporated. West Pharmaceutical Services is the exclusive distributor of Corning® Viridian® Vials.
REFERENCES
- Chard L, “Pharma CEOs write open letter calling suppliers to commit to sustainability”. CPHI Online, Jul 2023.
- Polasani S, “The Future of Sustainable Pharmaceutical Glass Packaging”. Company Web Page, Corning, accessed Sep 2024.

