To Issue 176
Citation: Allegro A, “Redefining Patient-Centric Drug Delivery: A Closer Look at Vertiva® On-Body Delivery System”. ONdrugDelivery, Issue 176 (Sep 2025), pp 36–39.
Anna Allegro explores current challenges in wearable injectors and how the next generation of on-body delivery systems, such as Stevanato’s Vertiva® platform, aims to close the gap between technical performance and true patient centricity.
Over the past decade, the drug delivery landscape has evolved rapidly to accommodate growing patient needs and therapeutic complexities. One significant advancement has been the emergence of on-body delivery systems (OBDSs), which have introduced greater flexibility and autonomy in managing chronic conditions outside clinical settings. These devices are gaining traction for their ability to simplify the administration of subcutaneous therapies, especially high-volume or high-viscosity drugs that would otherwise require frequent hospital visits or complex at-home procedures.
“AS MORE THERAPIES TRANSITION TOWARDS THE HOME ENVIRONMENT, PATIENTS ARE INCREASINGLY EXPECTED TO TAKE ON RESPONSIBILITIES TRADITIONALLY MANAGED BY HEALTHCARE PROFESSIONALS.”
However, the promise of wearable injectors has been accompanied by equally important questions surrounding usability, safety and patient adherence. As more therapies transition towards the home environment, patients are increasingly expected to take on responsibilities traditionally managed by healthcare professionals. This shift underscores the importance of intuitive, streamlined drug delivery solutions that minimise cognitive and physical burden. In this context, the user experience becomes not only a matter of comfort but a crucial factor in treatment adherence and overall outcomes.
MARKET LANDSCAPE: EVOLUTION OF OBDS DEVICES
Since 2016, six OBDSs have been introduced to the market:
- Repatha® Pushtronex® System (Amgen, CA, US, 2016): A 3.5 mL device delivering evolocumab for lowering LDL cholesterol.1
- Ultomiris® OBDS (Alexion Pharmaceuticals, MA, US, 2022): A 3.5 mL device for ravulizumab, used to treat paroxysmal nocturnal haemoglobinuria (PNH) and atypical haemolytic uremic syndrome.2
- Skyrizi® On-Body Injector (AbbVie, IL, US, 2022): A 3.5 mL device delivering 2.4 mL of risankizumab for plaque psoriasis, psoriatic arthritis and Crohn’s disease.3
- Furoscix® On-Body Infusor (scPharmaceuticals, MA, US, 2022): A 10 mL device administering furosemide for heart failure.4
- Empaveli® On-Body Injector (Apellis Pharmaceuticals, MA, US, 2023): A 20 mL device delivering pegcetacoplan for PNH.5
- Lasix® ONYU Infusor (SQ Innovation, MA, US, 2024): A 3 mL device that received the US FDA’s Tentative Approval for home treatment of fluid overload in congestive heart failure with 2.67 mL of furosemide. Final approval is still pending and market exclusivity for a competing product expires in October 2025.6
All these marketed OBDSs require either manual filling or loading of a drug-filled cartridge – a feature that increases complexity and introduces potential use errors during the critical moment of injection preparation. In fact, it is reasonable to expect that patients may feel the most stressed or nervous before treatment, which can make even simple steps difficult to perform accurately, thereby increasing the likelihood of errors. These challenges may have contributed to the discontinuation of some of these marketed user-loaded devices, with pharmaceutical providers acknowledging the importance of prioritising optimal patient experience.7,8 This calls for more patient-centric solutions that streamline the treatment process, minimise use steps, and enhance both safety and usability. Technologies such as Stevanato Group’s Vertiva® OBDS can play a role in addressing these gaps.
STEVANATO GROUP’S READY-TO-USE OBDS: VERTIVA®
Stevanato Group’s Vertiva® OBDS is a versatile device supporting programmable flow rates and injection times, making it suitable for basal infusions and bolus injections across diverse therapies, up to 10 mL in volume. Vertiva® features a single-use injection unit with a prefilled, preloaded drug cartridge and a reusable, multi-use smart controller connected via a proprietary magnetically-coupled drive mechanism (Figure 1). Its ready-to-use design significantly reduces complexity for users, allowing patients to begin treatment with greater ease and confidence while minimising potential use errors.
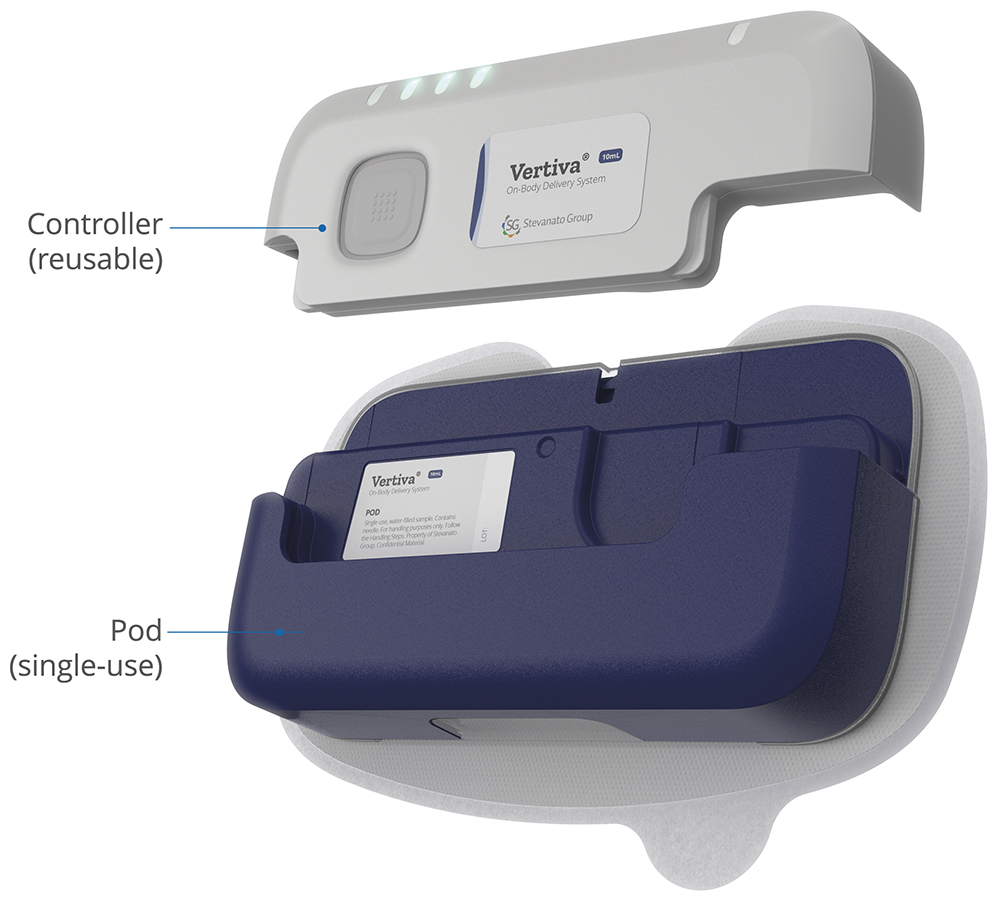
Figure 1: – Stevanato Group’s Vertiva 10 mL OBDS, featuring reusable electronics.

Figure 2: A patient completing the attribute assignation task during Stevanato Group’s patient preference study.
This advantage of Vertiva® has been observed in a patient preference study conducted by Stevanato Group in the UK in July 2024 (Figure 2). The study compared Vertiva® with a user-loaded OBDS available on the market, highlighting critical attributes that influence patient preferences for comparable delivery devices. Twelve participants, representing diverse demographics, medical conditions and varying levels of familiarity with injection devices, were involved. The study began with patients exploring the design and functionalities of the two OBDSs, watching an instructional video and reading summarised instructions for use. Patients were then asked to rank a list of pre-determined product attributes by importance, followed by assigning each attribute to the device they believed best fulfilled the criterion.
The study results highlighted that ease of administering treatment and the total number of use steps were the most critical attributes for patients. Notably, 10 out of the 12 patients considered it easier to administer treatment with Vertiva®, citing its preloaded and prefilled design, which reduces complexity and eliminates risks associated with manual cartridge loading, particularly during moments when patient stress and nervousness are at their peak.
Sustainability is another key focus of the Vertiva® system. By incorporating reusable electronics, it minimises environmental impact and offers cost-effective solutions for long-term treatments. This approach addresses growing environmental concerns while aligning with healthcare providers’ goals to adopt eco-friendly practices without compromising usability. In fact, while reusability adds some extra steps, such as the need for patients to store and possibly recharge the electronic unit, these occur after the injection, when patients are more at ease with their treatment.
“STEVANATO GROUP CAN PROVIDE PROPRIETARY PRIMARY PACKAGING SOLUTIONS, INCLUDING BULK
AND READY-TO-USE EZ-FILL® CARTRIDGES OPTIMISED FOR INTEGRATION WITH VERTIVA®.”
Another advantage for pharmaceutical manufacturers stems from Stevanato Group’s comprehensive end-to-end offering, which provides a variety of optional products and services to optimise Vertiva®’s supply chain. In addition to device development and manufacturing, Stevanato Group can provide proprietary primary packaging solutions, including ready-to-use EZ-fill® cartridges optimised for integration with Vertiva®. Additionally, Stevanato Group’s Technology Excellence Centers offer a suite of testing services, including drug-container-device interaction assessments. To further streamline operations, Stevanato Group also provides automated final assembly and packaging equipment. These capabilities are complemented by a strategic collaboration with a leading contract manufacturing organisation, Thermo Fisher Scientific (Waltham, MA, US).
THERMO FISHER AND STEVANATO GROUP PARTNERSHIP
In March 2023, Stevanato Group and Thermo Fisher announced a collaboration to offer an integrated, end-to-end solution to help streamline the supply chain management of the Vertiva® OBDS. Along with the proprietary device platform and the integrated services provided by Stevanato Group, Thermo Fisher will provide fill-finish and final assembly services (Figure 3). This has been supported with investment in a low-volume final assembly line at Thermo Fisher’s site in Ferentino (Italy) for final assembly of Vertiva® devices, with minimal changes required to the manufacturing equipment components when switching between the different device configurations.
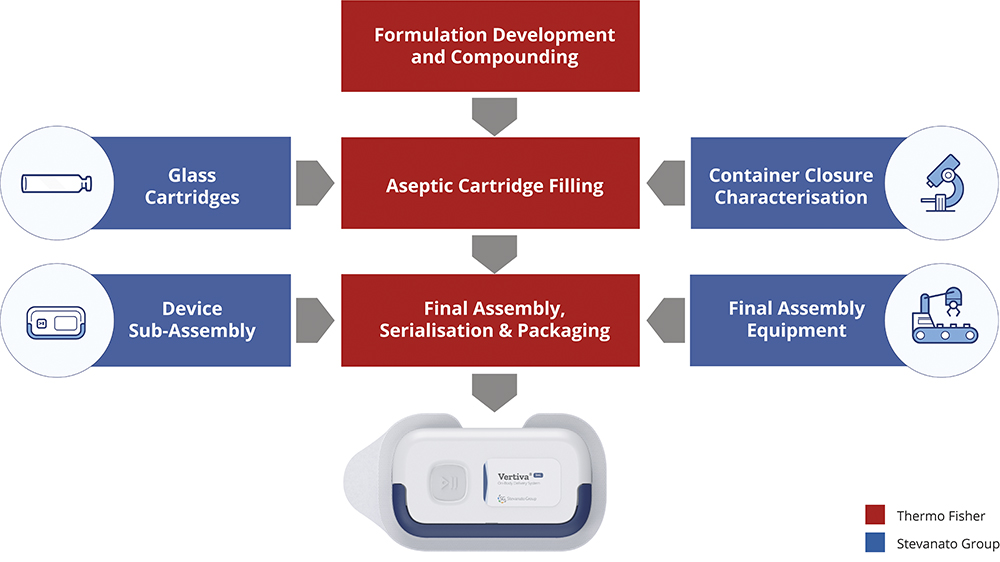
Figure 3: Stevanato Group and Thermo Fisher’s integrated Vertiva® offering.
“THE COLLABORATION BETWEEN THERMO FISHER AND STEVANATO GROUP IS DESIGNED TO ADDRESS CRITICAL CHALLENGES IN DRUG DELIVERY SYSTEM SUPPLY.”
The collaboration between Thermo Fisher and Stevanato Group is designed to address critical challenges in drug delivery system supply. By focusing on mitigating risks related to drug container compatibility and fill-finish techniques, the partnership ensures seamless integration with existing pharmaceutical workflows.Additionally, the partnership will simplify supply chain management, reduce operational complexity and allow pharmaceutical manufacturers to focus on innovation. Upfront preparation, work capabilities and scalable production solutions minimise customer investment, making the process more accessible and efficient. Ultimately, these advantages accelerate time-to-market, enabling companies to deliver therapies to patients more quickly, meeting urgent healthcare needs.
CONCLUSION
The future of drug delivery lies in advancing patient-centric, technology-driven solutions that align with modern therapeutic demands. OBDS platforms such as Vertiva® exemplify this progress, addressing critical challenges, such as larger-volume, high-viscosity and complex drug delivery while ensuring patient convenience and adherence. In this context, collaborative efforts between industry leaders, such as Stevanato Group and Thermo Fisher, will be vital in fostering innovation, optimising manufacturing processes and ensuring efficient supply chains for these transformative technologies.
REFERENCES
- “FDA approves first and only single monthly injection for a PCSK9 inhibitor”. Press Release, Amgen, Jul 2016.
- “Approval Letter for the New Drug Application (NDA) 761108”. US FDA, Jul 2022.
- “SKYRIZI® (risankizumab-rzaa) Receives FDA Approval as the First and Only Specific Interleukin-23 (IL-23) to Treat Moderately to Severely Active Crohn’s Disease in Adults”. Press Release, Abbvie, Jun 2022.
- “scPharmaceuticals Announces FDA Approval of FUROSCIX® (furosemide injection), the First and Only Self-administered, Subcutaneous Loop Diuretic for the At-home Treatment of Congestion in Chronic Heart Failure”. Press Release, scPharmaceuticals, Oct 2022.
- “Apellis Announces US FDA Approval of the EMPAVELI® Injector, a Device to Streamline Self-Administration”. Press Release, Apellis Pharmaceuticals, Oct 2023.
- “FDA grants Tentative Approval of SQ Innovation’s Lasix® ONYU”. Press Release, Gerresheimer, Dec 2024.
- “Repatha® Pushtronex® System (on-body-infusor with prefilled cartridge) Discontinuation – Frequently Asked Questions (FAQ)”. PDF, Amgen Inc, 2023.
- “AHUS Trial Watch: 18 Subcutaneous Ravulizumab”. Press Release, AHUS Alliance Action, Dec 2023.

