Richard Joye and Tim Morch discuss the advantages of using dissolvable microneedles for the delivery of vaccines, particularly in regards to storage and transport, and introduce SeriTech’s fast-detaching dissolvable microneedle technology, soon to be commercialised in the cosmetics sector under the brand name IPSYLON.
CHALLENGING THE HYPODERMIC MODEL
“Cold chain shipping and storage was valued at US$13 billion (£9.5 billion) in 2017 and has grown annually, especially since the creation of SARS-CoV2 vaccines. This complex system has a number of shortfalls, with as many as 40% of closed vial products being damaged prior to delivery.”
Vaccines remain one of the greatest developments of modern medicine. However, the 150-year-old hypodermic delivery model is undermined by cold chain logistics, dosage wastage, biohazardous waste and needlestick injuries.
Cold chain shipping and storage was valued at US$13 billion (£9.5 billion) in 2017 and has grown annually, especially since the creation of SARS-CoV2 vaccines. This complex system has a number of shortfalls, with as many as 40% of closed vial products being damaged prior to delivery. In 2005, it was estimated that, annually, over 50% of vaccines are wasted worldwide. The WHO recently launched three new workstream programmes to address this issue, measuring effective vaccine management, cold chain equipment and optimisation.
With the current global focus on covid-19 vaccines, ultra-cold freezers, specialised syringes and licensed administrators, cracks in the traditional system are beginning to appear. The impact has been most evident on developing nations.
These vaccine programmes are expensive and time consuming – the urgency to develop a new solution has never been greater. With a clear unmet need in the market, dissolving microneedle (dMN) technology has the potential to transform the current landscape.
REDUCING COSTS, INJURIES AND WASTE
dMN technology offers a proven alternative to traditional vaccine delivery methods, reducing the need for cold chain logistics and shipping, and thereby lowering storage and transport costs. dMNs also eliminate the biohazardous waste and needlesticks injuries associated with hypodermic needles, as well as reducing the impact of drug waste issues and dose sparing. Furthermore, dMNs provide improved vaccine stability, reduction of vaccine wastage and of burden on trained personnel. These benefits in particular make dMN vaccines an attractive option for use in developing countries.1
DISSOLVING MICRONEEDLES FOR VACCINES
“In contrast to traditional hypodermic syringes, influenza vaccines formulated as dMNs show strong thermostability. dMN patches can retain influenza vaccine activity outside of cold chain storage, as well as when subject to other environmental stresses.”
Vaccines prevent millions of deaths every year. Over 6.4 billion flu vaccines are administered annually, and the explosion of covid-19 vaccines will be added to this list. Most vaccines are sensitive to variations in temperature and need to be maintained in strictly controlled cold chain up to delivery. This represents challenges, particularly for last-mile delivery.
In contrast to traditional hypodermic syringes, influenza vaccines formulated as dMNs show strong thermostability. dMN patches can retain influenza vaccine activity outside of cold chain storage, as well as when subject to other environmental stresses. With this in mind, it isn’t a significant leap to consider the possibility of a dMN vaccine that can be safely stored on a shelf in a clinic or pharmacy.2
When compared with vaccinations delivered by traditional hypodermic syringes in preclinical studies, dMN-based vaccines have demonstrated comparable antibody and cellular responses. In some cases, these responses were in fact higher and more durable than the conventional vaccines.1 As such, dMN technology is likely to lead to a significant shift in how vaccines are manufactured, shipped and administered.
From a patient perspective, dMNs represent a safe, simple and painless method of delivering vaccines. Most importantly, dMNs deliver medicines more efficiently and equitably.
THE SERITECH SOLUTION
“SeriTech develops products leveraging a unique technology, the world’s first fast-detaching dMN patch for a safe, effective and affordable alternative to traditional drug delivery. Transdermal patch products from SeriTech can be applied in just two minutes.”

Figure 1: The SeriTech fast detaching dissolving microneedle patch.
SeriTech creates innovative, effective health and skin care products for the 21st century, operating in three core areas – medicinal, therapeutic, and cosmetic products. SeriTech develops products leveraging a unique technology, the world’s first fast-detaching dissolving microneedle (fddMN) patch for a safe, effective and affordable alternative to traditional drug delivery (Figure 1). Transdermal patch products from SeriTech can be applied in just two minutes.
The SeriTech solution doesn’t suffer from the limitations of many other dMN patches, with longer application times ranging from two to six hours. This technological breakthrough avoids shifting patches, skin irritation and related complications that longer application times can incur.
SeriTech is also evaluating how silk proteins could improve transdermal drug delivery, capitalising on the company’s innovative, proprietary process to produce pharmaceutical grade fibroin and sericin. The next generation of fddMN patches will use silk proteins, with the combination being a unique IP. Silk is an attractive material as it is intrinsically non-toxic, biocompatible and biodegradable, self-assembling, mechanical stable and structurally controllable.3
Sericin inhibits inflammation and has antibacterial, antioxidant, anti-coagulative and regenerative properties, and Fibroin can increase the structural strength of dMNs. Insulin delivery research has established that arrays composed of silk fibroin tips have robust mechanical strength and can achieve rapid dissolution and drug release. Furthermore, silk fibroin enhances the stability of enzymes and proteins, with insulin-loaded dMNs having a shelf life of 20 days at room temperature.4
IPSYLON: DERMOCOSMETIC SKINCARE PRODUCTS

Figure 2: IPSYLON fast detaching dissolving microneedle patch.
SeriTech is bringing its revolutionary patch to market with the launch of IPSYLON (Figure 2). SeriTech’s skincare brand will be available starting Q4 2021 in Spain and then in selected markets, following the successful completion clinical tests. Proving this technology in commercial applications will pave the way for its use as a drug delivery device in the healthcare sector, for vaccines and other drugs.
IPSYLON dermocosmetic skin care products offer a suite of transdermal micro-vectors that deliver the active ingredient exactly to the target site. SeriTech and IPSYLON were born of company founder Saimai Cunvong’s desire to capitalise on new technologies to deliver improved products.
Each of IPSYLON’s fddMN patches are designed for a specific purpose. The products are minimalist, have no additives and contain a blend of hyaluronic acid, vitamin C, and retinal.
IPSYLON eliminates lengthy skincare routines by providing easy, fast and effective skin treatment. Different patch shapes fit the target areas – curved for around the eyes or nasolabial areas or round for dark spots and acne scars. IPSYLON provides professional quality skincare that is easily applied at home or on the fly.
IPSYLON targets wrinkles associated with ageing, dark spots, and acne scars. Product ingredients are specifically blended to hydrate and rejuvenate, increasing elasticity and nourishing the skin. Polymeric tip arrays vary in number depending on product, target area and load blend. The “Dark Spot Corrector” and “Acne Scar Smoother” feature 137 dMNs, the “Deep Filler” comprises of 557 hybrid long dMNs, and the “HA Intense” and “Retinal Filler” have 607 dMNs for fine line and wrinkle reduction.
Laboratory Results
Laboratory in vivo analysis validates the ease of use of SeriTech’s fddMNs and demonstrates the efficacy of IPSYLON products, which have been certified by Bionos Biotech (Valencia, Spain) to meet EU Good Clinical Practice (GCP) regulations and qualify as “Dermatologically Tested”, “Clinically Tested” and “Tolerance Tested” products.
Results confirm the Dark Spot Corrector significantly decreases the area for dark spots and increased skin uniformity. Retinal Filler patches display anti-wrinkle capabilities in nasolabial area, demonstrating a significant reduction in the total area, length, depth and roughness of the skin, and a significant increase in smoothness.
Acne Scar Smoother Patches display anti-rugosity capabilities and increase skin smoothness, with a significant reduction in the roughness of the area showing acne scars, compared with the basal values before the start of the treatment.
HOW SERITECH’S FDDMN WORKS
SeriTech’s fddMNs are safe and easy to apply. The patch, consisting of a micro-array of tips and a base, is configured to best fit the target area and deliver the load. An IPSYLON patch has two faces – the front, where the dMNs are, and the back where the wet detach pad is applied. During application it is important to never touch the dMNs on the front of the device.
To use an IPSYLON patch, follow these steps (Figure 3):
- Gently open the sterile container
- Thoroughly wash the skin around the target area
- Extract the IPSYLON patch from the sterile container
- Make sure which side of the IPSYLON patch is the front and which is the back
- Hold the IPSYLON patch by the rim so as not to touch the dMNs on the front
- Peel away the plastic protection sheet from the IPSYLON patch
- Apply the IPSYLON patch to the target area thoroughly and press for 30 seconds
- Place the wet detach pad on the back of the IPSYLON patch and press for two minutes to allow the dMNs to detach from the patch.
After application, remove the base of the IPSYLON patch and use the same detach pad to gently massage the area to facilitate the penetration and dissolution process.
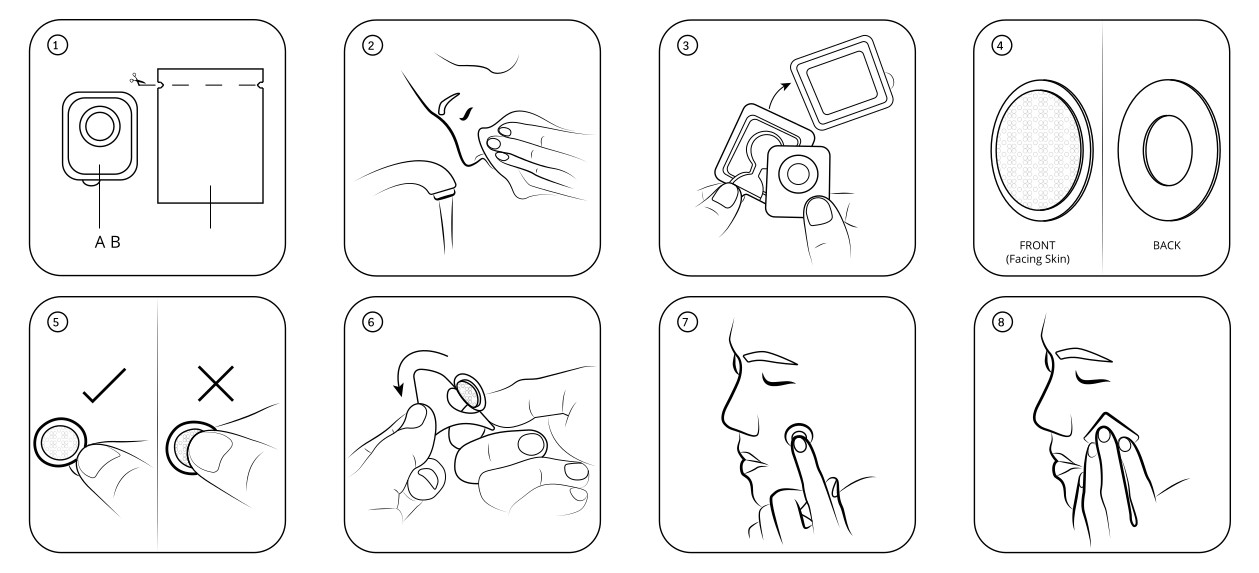
Figure 3: How to apply the SeriTech/IPSYLON fast detaching dissolving microneedle patch.
“The company is investigating how to integrate silk proteins as polymeric blends in its fddMNs. Additional research includes potential applications for innovative treatments for indications including cancer, glaucoma, diabetes, Alzheimer’s and asthma.”
SERITECH R&D
The R&D team at SeriTech is investigating a range of pharmaceutical applications for its fddMN technology, focusing on vaccines. The company is investigating how to integrate silk proteins as polymeric blends in its fddMNs. Additional research includes potential applications for innovative treatments for indications including cancer, glaucoma, diabetes, Alzheimer’s and asthma.
The advantages of dMNs compared with other systemic administration methods could be appealing in other biomedical applications.5 Cancer research indicates that transdermal microneedle-based drug delivery can delay tumour initiation and slow tumour growth in a therapeutic model.6 SeriTech anticipates increased collaboration with scientists to deliver these medicines via its fddMN platform.
REFERENCES
- Leone M et al, “Dissolving Microneedle Patches for Dermal Vaccination”. Pharm Res, 2017, Vol 34, pp 2223–2240.
- Mistilis MJ et al, “Long-term stability of influenza vaccine in a dissolving microneedle patch”. Drug Dev Transl Res, 2016, Vol 7, pp 195–205.
- Veiga A et al, “Recent Advances in Silk Sericin/Calcium Phosphate Biomaterials”. Front Mater, 2020, Vol 7(24), online.
- Lau S et al, “Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery”. J Control Release, 2017, Vol 265, pp 113–119.
- Yang J at al, “Recent advances of microneedles for biomedical applications: drug delivery and beyond”. Acta Pharm Sin B, 2019, Vol 9(3), pp 469–483.
- AA Ali et al, “DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles”. Nanomedicine: NBM, 2017, Vol 13(3), pp 921–932.

