To Issue 160
Citation: Berger L, Linvill E, “SHL Molly® Autoinjectors: Powering the Next Wave of Cardiometabolic Care”. ONdrugDelivery, Issue 160 (May 2024), pp 42–46.
Lars Berger and Eric Linvill discuss the rapid growth of the cardiometabolic disease space in light of the global obesity epidemic and how advanced autoinjector and injection pen technologies, such as SHL Medical’s Molly platform, will be necessary to meet the challenges presented by this disease area.
The rapid growth of glucagon-like peptide-1 receptor agonists (GLP-1RA) for the treatment of obesity has drawn public attention to treatments for cardiometabolic diseases. With an already significant demand for such treatments that is expected to grow further, the interest in new drug development in this area is increasing. Most of these medications require injectable formats, creating a need for user-friendly autoinjector devices that are readily available to the pharmaceutical industry on a large and unprecedented scale.
“Now recognised as an epidemic, there is increasing pressure on hospitals and clinics, as the centre of care, to treat obesity and other related diseases medically.”
ADDRESSING THE BURDEN OF OBESITY AND OTHER METABOLIC DISEASES
The pervasiveness of obesity continues to be staggering. According to the latest available data published in The Lancet, more than a billion people worldwide are living with obesity. In the US alone, the 2022 adult prevalence rate for obesity ranged from 37.7–47.7%. Global estimates for the year 2035 show that no apparent reversal of the trend is coming, with 1.9 billion people expected to be affected. Now recognised as an epidemic – referred to as “globesity” by the WHO – there is increasing pressure on hospitals and clinics, as the centre of care, to treat obesity and other related diseases medically.1–4
In addressing this global epidemic, it is important to acknowledge the relationship between obesity and other cardiometabolic diseases. For this, one is reminded of the strong link between obesity and metabolic syndrome (MetS). In the words of the American Heart Association and the National Heart, Lung, and Blood Institute, MetS is a “constellation of interrelated risk factors of metabolic origin”. Such cardiometabolic risk factors, the most widely recognised of which include dyslipidaemia, hypertension and elevated plasma glucose, have been shown to promote the development of atherosclerotic cardiovascular disease (ASCVD). At the same time, MetS has been reported to increase the risk of Type 2 diabetes (T2D).5–8
The clear links between MetS, its underlying risk factors and resultant diseases such as ASCVD and T2D open many pathways to treating such cardiometabolic conditions clinically, together or individually. As such, it becomes increasingly clear that treatment delivery must go beyond the contemporary focus on obesity and recognise the network of interrelated cardiometabolic diseases that lie in front.
As preventative care has shown little success in halting the rise in cardiometabolic diseases, new medicines will be crucial for improving the quality of life and independence of as many patients as possible. As research and development in GLP1-RAs and other cardiometabolic treatments continues to evolve, so too does the opportunity to enable at-home patient care with pen injectors and autoinjectors. At-home drug delivery opens pathways to unburden primary care services and enables treatment strategies in safe and easy-to-use self-injection formats. For pharma and biotech companies with cardiometabolic compounds in the pipeline, selecting the right device partner is critical to ensuring a successful launch and continued success.
A HIGH FUTURE GROWTH FOR INCRETIN MIMETICS AND AT-HOME DRUG DELIVERY
Progress in the science of GLP1-RAs has come a long way (Table 1). Since the isolation of glucose-dependent insulinotropic polypeptide (GIP) in the 1970s, further progress has been made in incretin biology, leading to the discovery of the physiological actions of GLP-1 receptor agonists. However, it was only in 2012 – roughly a decade ago – that once-weekly GLP-1RAs were launched. This started with the launch of the long-acting/extended release exenatide microspheres, followed by dulaglutide and albiglutide in 2014.10 Interestingly, perhaps the most successful of this wave of once-weekly products, dulaglutide, was launched in a three-step autoinjector, which compared favourably with formulations that required more complicated preparation steps for the user.
| GLP1-RA | Date of First Approval | Elimination Half-Life |
Administration Schedule |
Pharmaceutical Company |
| SUBCUTANEOUS INJECTIONS | ||||
| Short-acting compounds | ||||
| Exenatide b.i.d | 2005 (US); 2006 (Europe) | 3.3–4.0 hrs | Twice daily | AstraZeneca |
| Lixisenatide | 2013 (Europe); 2016 (US) | 2.6 hrs | Once daily | Sanofi |
| Long-acting compounds | ||||
| Lixisenatide | 2009 (Europe); 2010 (US) | 12.6–14.3 hrs | Once daily | Novo Nordisk |
| Exenatide once weekly | 2012 | 3.3–4.0 hrs | Once weekly | AstraZeneca |
| Dulaglutide | 2014 | 4.7–5.5 days | Once weekly | Eli Lilly and Company |
| Albiglutide | 2014 (Europe & US) | 5.7–6.8 days | Once weekly | GlaxoSmithKline |
| Semaglutide | 2017 (US); 2019 (Europe) | 5.7–6.7 days | Once weekly | Novo Nordisk |
| Fixed-dose combinations | ||||
| Liraglutide & Insulin degludec |
2014 (Europe); 2016 (US) | 12.6–14.3 hrs | Once daily | Novo Nordisk |
| Lixisenatide & Insulin glargine |
2016 (US); 2017 (Europe) | 2.6 hrs | Once daily | Sanofi |
| ORAL ADMINISTRATION | ||||
| Long-acting compound | ||||
| Semaglutide | 2020 | 5.7–6.7 days | Once daily | Novo Nordisk |
Table 1: A non-exhaustive list of GLP1-RAs that have been approved to treat T2D. Elimination half-lives were adapted from a 2021 review paper by Nauck et al. The list does not include compounds that are still in development or where available information is scarce, such as efpeglenatide, beinaglutide and PEG-loxenatide. Exenatide was also launched as a once-weekly autoinjector in 2018, marketed as BYDUREON® BCise™ (AstraZeneca).9,10,11
“Since 2015, SHL has supported the launch of six combination products indicated for cardiometabolic diseases, including incretin mimetics for T2D and obesity in particular, as well as PCSK9 inhibitors for hyperlipidaemia.”
Not long after, the therapeutic impact of GLP1-RAs expanded from diabetes to chronic weight management for adults with obesity. Key compounds that come to mind are semaglutide, marketed as Wegovy® by Novo Nordisk, and Eli Lilly’s tirzepatide, marketed as Zepbound®. The further enhanced effects of these second-generation compounds on lowering blood glucose, reducing weight, and protecting the heart and kidneys, is now encouraging further clinical development and points to many possible novel combinations of medicines in the future.
TREATMENT OF CARDIOMETABOLIC DISEASES ASKS FOR SPECIFICALLY DESIGNED DELIVERY DEVICES
Today, there are more than two dozen therapeutic candidates in the GLP-1 drug class undergoing clinical research. Depending on the API and the formulation, these drugs require a variety of delivery devices, ranging from multi-dose pen injectors to syringe- and cartridge-based autoinjectors. Additionally, these treatments are being developed for conditions such as obesity, diabetes and metabolic dysfunction-associated steatohepatitis (MASH), where patients may potentially require the simultaneous use of multiple medications. Each medication, in turn, requires a specific delivery device technology.12
Reflecting on the success of already-marketed devices, it is evident that future cardiometabolic treatments will rely in large part on proven drug delivery technologies, like two-step single-use autoinjectors. Whether for novel compounds or generics, such molecules will require user-friendly self-injection solutions developed by a device partner with proven expertise in producing devices for this emerging disease area.
A DECADE OF EXPERIENCE IN CARDIOMETABOLIC DISEASES
With more than 30 years of industry experience in total – resulting in more than 50 combination products – the last ten years has seen SHL tackling a plethora of device projects in collaboration with pharma companies, particularly in the cardiometabolic disease area. Since 2015, SHL has supported the launch of six combination products indicated for cardiometabolic diseases, including incretin mimetics for T2D and obesity in particular, as well as PCSK9 inhibitors for hyperlipidaemia.
In so doing, SHL delivered at least 49.5 million devices into the hands of patients living with cardiometabolic diseases in 2023 alone, a number unrivaled in the field of proprietary autoinjector technology. Further analysis of IQVIA data suggests that SHL’s devices have supported the treatment journey of at least 1.6 million patients in this therapeutic area (Figure 1).
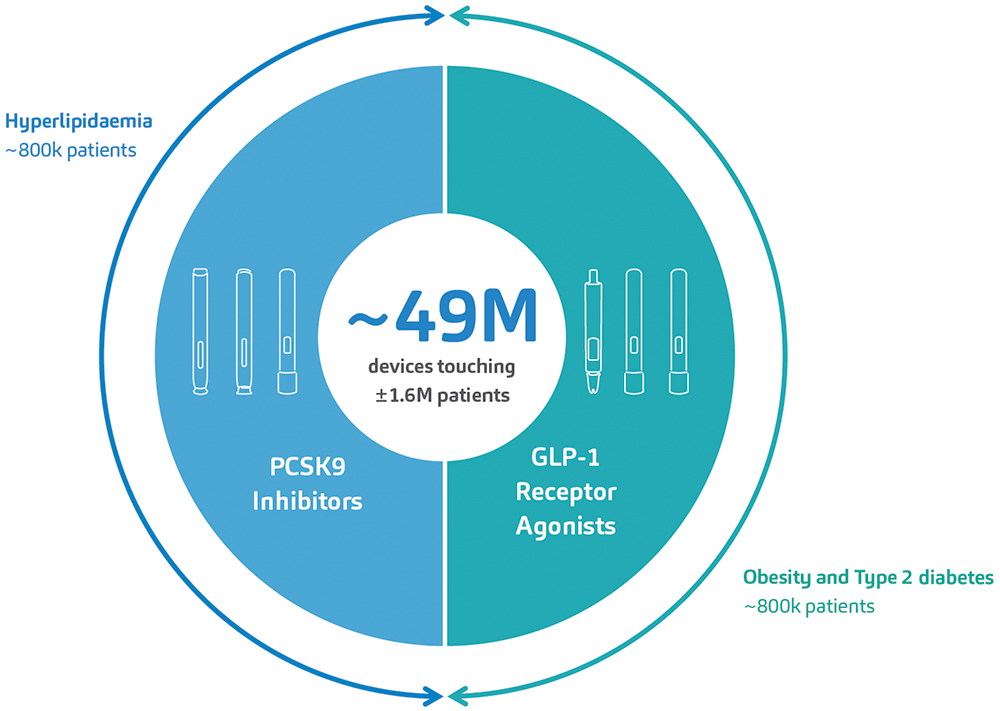
Figure 1: SHL’s devices have supported the launch of six combination products for the treatment of cardiometabolic diseases within the last 10 years. An analysis of market data by IQVIA indicates that SHL’s devices have touched at least 1.6 million patients afflicted with hyperlipidaemia, T2D or obesity. These numbers do not include emergency combination product treatments for severe hypoglycaemia.
“With the obesity epidemic and the rising prevalence of cardiometabolic diseases, understanding adipose tissue function and distribution becomes critical.”
Many of these combination product launches relied on SHL’s modular Molly platform autoinjectors (Figure 2). In drug-device projects within the cardiometabolic area, the demand is often high. Pharma and biotech companies need partners with proven device technologies supported by a globally dispersed infrastructure capable of scaling manufacturing. They also need to ensure high satisfaction rates for end users. SHL’s Molly autoinjector has consistently met this challenge – the modular platform has been integral to the second-generation GLP1-RAs approved for T2D (in Japan in 2020) and obesity (in the US in 2021).13,14

Figure 2: Built upon proven experience in the cardiometabolic space, SHL’s modular Molly platform autoinjectors are well positioned to address the up-and-coming developments in this therapy area.
The Molly platform has also proven itself in the lifecycle management of product families. In 2015, one of SHL’s pharma partners decided to perform lifecycle management on its PCSK9 autoinjectors for hyperlipidaemia. The project required two dosing formats in autoinjectors bearing communal industrial design and branding elements set by the pharma partner. A published usability study on the now-marketed high-dose, 2.0 mL format reported “no new product technical issues or no new safety concerns” compared with the 1.0 mL autoinjector format marketed previously. Study results like this affirm SHL’s original findings on the Molly device’s ease of use and highlight the platform device’s applicability in metabolic therapy areas.15
Today, the metabolic space is experiencing extraordinary growth, with emerging clinical progress in mono-, dual- or triple-receptor agonists for obesity. The Molly autoinjector is well positioned to address emerging candidate compounds in this burgeoning market, offering quick-to-clinic device development options to support a pharma company’s clinical trial strategy.16
SHL’S COMMITMENT TO PATIENTS
As SHL continues to collaborate with global pharma companies across various treatment areas, its commitment to providing the best possible experience for end users of its autoinjector technologies remains steadfast. As pioneers in self-injection technology, one of SHL’s aims is to deepen its understanding of how autoinjectors activate, even in the softest injection sites.
Various studies have shown that body mass index (BMI) does not sufficiently reflect adipose tissue distribution, which includes visceral fat. With the obesity epidemic and the rising prevalence of cardiometabolic diseases, understanding adipose tissue function and distribution becomes critical. This is increasingly relevant for disease areas such as obesity and cardiometabolic diseases, where metabolic phenotypes could differ from other patient populations.17–19
To address this, the SHL research team conducted a series of soft tissue user (in vivo) studies, simulation (in silico) studies and physical test (in vitro) studies, providing a mechanistic view of the interactions between the autoinjector and human soft tissue during injection activation (Figure 3). The development of these in silico and in vitro models, which themselves represent challenging cases from the in vivo studies, goes above and beyond what is required in standard autoinjector testing methodologies, affirming SHL’s commitment to end users. This study on autoinjector activation performance also confirms the safety and reliability of SHL’s autoinjector technologies, even in more complex or heterogeneous patient groups.
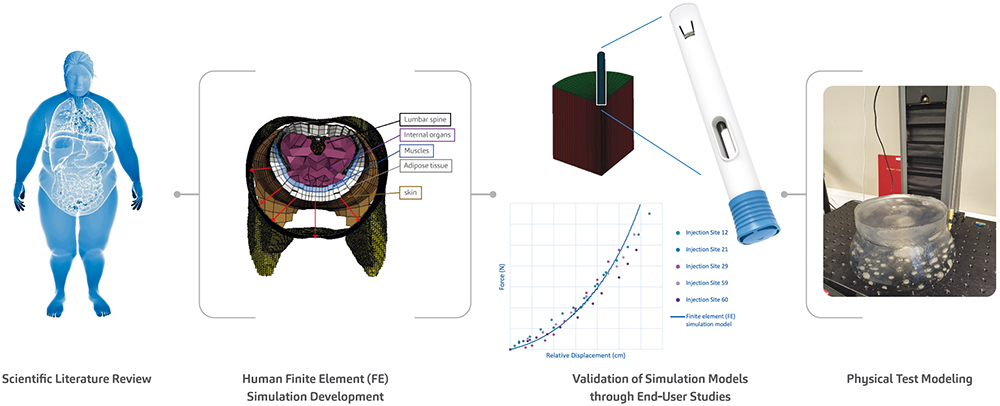
Figure 3: As part of SHL’s efforts to advance the understanding of autoinjector activation even in the softest injection sites, SHL’s research team presented the proceedings of a study at the 2023 Parenteral Drug Association (PDA) Universe of Pre-Filled Syringes and Injection Devices Conference.20
ADDRESSING CARDIOMETABOLIC CHALLENGES WITH INNOVATIVE SOLUTIONS
The present obesity epidemic, affecting more than a billion people, underscores the urgency of addressing the various branching healthcare challenges related to cardiometabolic diseases. SHL Medical, a leader in self-injection solutions, is dedicated to improving lives with its autoinjector technologies. With a proven track record of meeting the growing needs of the cardiometabolic market, SHL’s Molly autoinjectors are poised to usher in a new era in cardiometabolic care. SHL is committed to responding with innovative solutions to meet emerging needs for specialised device technologies in future drug discoveries.
REFERENCES
- “Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population representative studies with 222 million children, adolescents, and adults”. Lancet, 2024, Vol 403(10431), pp 1027–1050.
- “Body-Mass Index – Evolution of BMI over time”. Web Page, NCD RisC, accessed May 2024.
- “World Obesity Atlas 2023”. World Obesity Federation, Mar 2023.
- “Controlling the global obesity epidemic”. Web Page, WHO, accessed May 2024.
- Grundy SM et al, “Diagnosis and Management of the Metabolic Syndrome”. Circulation, 2005, Vol 112(17), pp 2735–2752.
- Lusis AJ, Attie AD, Reue K, “Metabolic syndrome: from epidemiology to systems biology”. Nat Rev Genet, 2008, Vol 9(11), pp 813–830.
- Cornier M-A et al, “The metabolic syndrome”. Endocr Rev, 2008, Vol 29(7), pp 777–822.
- Fahed G et al, “Metabolic Syndrome: Updates on Pathophysiology and Management in 2021”. Int J Mol Sci, 2022, Vol 23(2), Article 786.
- Nauck MA “GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art”. Mol Metab, 2021, Vol 46, Article 101102.
- Andersen A et al, “Glucagon-like peptide 1 in health and disease”. Nat Rev Endocrinol, 2018, Vol 14(7), pp 390–403.
- Wang J-Y et al, “GLP−1 receptor agonists for the treatment of obesity: Role as a promising approach”. Fron Endocrinol (Lausanne), 2023, Vol 14, Article 1085799.
- Internal analysis of trials involving GLP-1 RA and GLP-1 RA combinations in Phases II and III registered on clinicaltrials.gov. SHL, February 2024.
- “2型糖尿病治療剤 持続性GLP-1 受容体作動薬/セマグルチド(遺 伝子組換え)オゼンピック® 皮下 注 0.25mg SD、0.5mg SD、1.0mg SD – 新発売のご案内” (Type 2 diabetes treatment agent Long-acting GLP-1 receptor agonist/semaglutide (genetical recombination) Ozempic® subcutaneous injection 0.25mg SD, 0.5mg SD, 1.0mg SD – New release information). Japanese Press Release, Novo Nordisk, Jun 2020.
- “FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014”. Press Release, US FDA, Jun 2021.
- Frias JP et al, “The SYDNEY Device Study: A Multicenter, Randomized, Open-label Usability Study of a 2-mL Alirocumab Autoinjector Device”. Clin Ther, 2020, Vol 42(1) pp 94–105.
- Jastreboff AM, Kushner RF, “New Frontiers in Obesity Treatment: GLP-1 and Nascent Nutrient-Stimulated Hormone-Based Therapeutics”. Annu Rev Med, 2023, Vol 74, pp 125–139.
- Mittal B, “Subcutaneous adipose tissue & visceral adipose tissue”. Indian J Med Res, 2019, Vol 149(5), pp 771–573.
- Goossens GH, “The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function”. Obes Facts, 2017, Vol 10(3), pp 207–215.
- Shuster A et al, “The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis”. Br J Radiol, 2012, Vol 85(1009), pp 1–10.
- Linvill E et al, “Rethinking human factors in obesity: Development of simulation and physical test models of human soft tissue to study autoinjector activation performance”. Poster Presentation, PDA Universe of Pre-Filled Syringes and Injection Devices Conference, Gothenburg, 2023.

