To Issue 154
Citation: Berg C, Chin W, Goncalves P, “Smart Scaling for Successful Capsule-Based Dry Powder Inhaler Filling Using Drum Technologies”. ONdrugDelivery, Issue 154 (Nov 2023), pp 28–32.
Carolyn Berg, William Chin and Patrick Goncalves discuss the considerations for filling capsules for dry powder inhalers across the various stages of clinical trials and commercialisation, and how to tackle the challenges of scaling up filling processes.
Over the past two decades, the field of respiratory medicine has undergone significant growth and evolution. What was once a focus on developing drugs to treat asthma and chronic obstructive pulmonary disease (COPD) has expanded to include a range of therapy areas, including the respiratory, anti-infective, neurological and cardiovascular sectors, among others (Figure 1). According to industry clinical trial databases, nearly 100 molecules for respiratory delivery are currently in the clinical development pipeline, spanning from preclinical to Phase III stages, for both pulmonary and non-pulmonary indications.1 The growing interest in targeting the lungs as a viable drug delivery route reflects the potential of this approach to improve the treatment of a wide range of diseases.
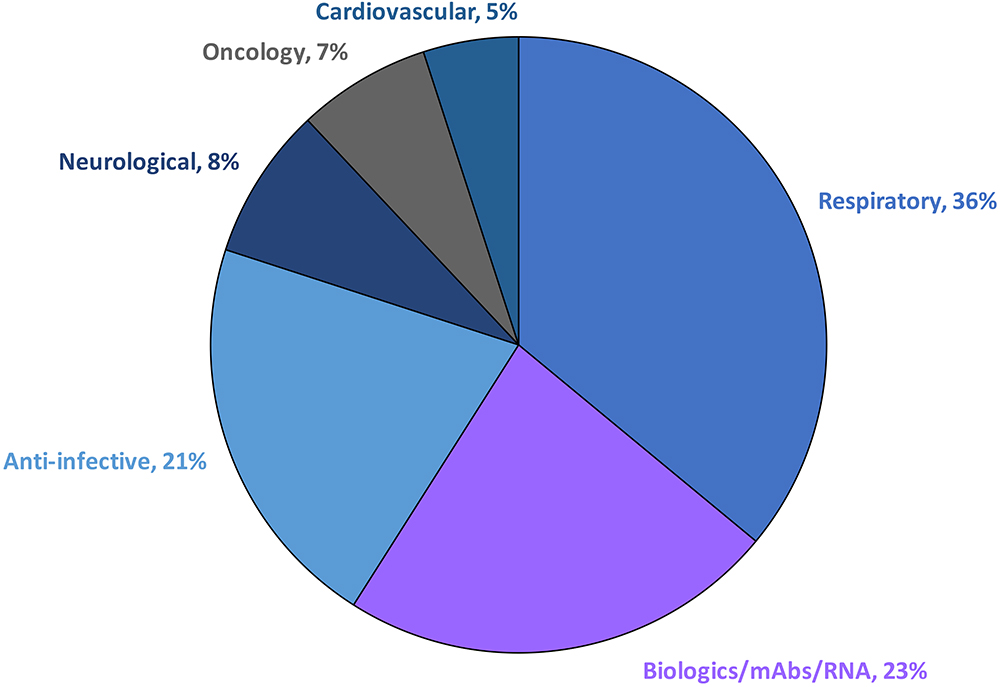
Figure 1: Pulmonary drug delivery sector by therapy area. Pulmonary delivery is a growing field with the potential to revolutionise the treatment of a wide range of diseases.
“DPIs are easy to use and their propellant-free design makes them a safe and convenient choice for individuals across age groups.”
By targeting the lungs directly, the pulmonary route of administration can minimise the systemic side effects often associated with other delivery methods. Traditionally, large doses of inhaled drugs have been administered by nebulisers. However, these devices are inconvenient for patients, as they are bulky, noisy, require a power source and have a longer administration time than other inhalation devices.2 On the other hand, dry powder inhalers (DPIs) are easy to use and their propellant-free design makes them a safe and convenient choice for individuals across age groups. DPIs are based on the concept of a dose metering system that delivers a determined dose to the patient.
Various dose metering systems, such as single-dose capsule-based DPIs (sDPIs), blister-based devices and reservoir-based devices, each offer unique advantages and challenges.3 Furthermore, they provide a good delivery method by offering targeted lung delivery with reduced side effects.4 While blister- and reservoir-based systems offer advantages for specific applications and patient needs, sDPIs are generally considered to be the most versatile and user-friendly type of DPI due to two important factors:
- Different-sized capsules, ranging from Size 00 to Size 3, can be used.
- Different fill weights per capsule can be achieved, depending on the manufacturing technique of the powder and the variability of the fill weight per capsule.
Typically, sDPIs devices use a capsule containing the drug product, which is punctured to release the drug upon inhalation. Most inhalable drugs in development use hydroxypropyl methylcellulose (HPMC) capsules due to their high moisture barrier and lower risk of breakage upon puncture, thereby ensuring effective drug release.
However, manufacturing sDPIs presents its own set of challenges, including meticulous control over capsule materials and dimensions, as well as powder characteristics. The capsule’s design must facilitate puncturing with a specific force and ensure uniform powder release. The performance also depends on the capsule’s material, the inhaler design and the powder formulation contained within. This last point is the most crucial, as the flow characteristics of the powder can vary depending on the type of powder formulation. Each type of powder, whether it be a blended, carrier-based product with micronised drug or an engineered particle obtained through spray drying, has a unique set of rheological properties that can have a significant impact on the downstream manufacturing processes involved in capsule filling.5
FACTORS TO CONSIDER WHEN SELECTING CAPSULE-FILLING TECHNOLOGY FOR DPIs
When it comes to filling capsules for DPIs, there are two primary methods to choose from – drum filling and dosator-based filling. It is important to determine which dosing system is most suitable for the given powder formulation as early as possible during product development so as to reduce the risk of needing to change the manufacturing process during clinical trials and commercialisation.
Drum filling is a method that uses a rotating drum to transfer powder into the capsules, with a vacuum capability to draw in the powder. On the other hand, dosator-based capsule filling uses a precise metering device called a dosator to dispense the powder into the capsules. Both filling methods are highly accurate, but drum filling has an edge over dosator-based filling due to its superior accuracy. Drum filling has also proved to be very robust across a range of powder properties,6 making it a reliable option for precise dosing, especially for high-dose formulations. Additionally, drum filling is suitable for low-potency, high-dose drugs.7 Both methods can fill a wide range of capsule sizes and formulations, but dosator-based filling has some limitations compared with drum filling (Table 1).
| Feature | Drum Filling | Dosator-Based Filling |
| Principle |
|
|
| Dosing range |
|
|
| Accuracy |
|
|
| Flexibility |
|
|
| Powder characteristics |
|
|
| Equipment complexity |
|
|
| Residual volume |
|
|
Table 1: Comparison of drum filling and dosator-based filling for sDPIs.
Choosing between a dosator or a drum system for filling capsule-based DPIs with spray-dried powder depends on specific project needs and requirements. One key factor to consider is the flowability of the powder, as it determines which filling system is most suitable. Powders with a flow function coefficient (FFC) of less than four can be filled using a dosator system, while those with an FFC greater than four are more compatible with a drum system.8 Another important consideration is the strength of the vacuum in the drum system, which has a significant effect on the fill weight. If there is a need to fill many capsules quickly and efficiently, vacuum-based drum filling is a good option. According to the data presented in Table 2, it can be observed that, in the case of most approved spray-dried products available in the market, drum-based filling is the prevalent technology used for capsule filling.
| Molecule | Product | Company | Technology | ||
| Manufacturing | Filling | Device | |||
| Insulin | Exubera®*2 | Nektar | Spray dried | Drum | Unit dose blisters Proprietary inhaler |
| Insulin | Afrezza® | Mannkind | Freeze-dried Technosphere® | Drum | Unit dose blisters Proprietary inhaler |
| Treprostinil | Tyvaso DPI® | United Therapeutics & Mannkind | Spray dried | Drum*** | Single-dose cartridge Dreamboat® |
| Levodopa | Inbrija®**9 | Acorda | Spray dried | Drum (inferred) | Capsule Proprietary inhaler (pen device) |
| Tobramycin | Tobi®10 | Novartis | Spray dried | Drum | Capsules Podhaler® |
*Withdrawn in 2007 for commercial reasons
**Original patents suggest dosator technology, but manufacturing facility supports drum technology
***Uses same device and technology as Afrezza
Table 2: Commercialised spray-dried inhalable powders.
“One of the significant challenges in the process is scaling up capsule filling for clinical trials, which requires comprehensive planning and execution.”
ADVANCED DOSING SYSTEMS FOR CAPSULE-BASED DPIs
Catalent’s state-of-the-art facility is equipped with two advanced compact benchtop dosing devices – the Drum TT and Drum Lab – both of which are capsule-filling machines leveraging the latest drum dosing technology from Harro Höfliger. The Drum TT, a compact manual filling machine, can be conveniently placed on any table top. It can precisely measure and fill as little as 0.5 mg of material into capsules within a short period of time. Additionally, it features a sophisticated pneumatic control system to regulate vacuum and compressed air. In the initial stages of clinical development, the Drum TT can be used to prepare galenic formulations for capsule-based DPIs. It is particularly useful for testing different powder formulations and optimising them for batch manufacturing.
The Drum Lab system is designed to semi-automatically dose small quantities of powder into capsules. This equipment is suitable for small-batch production, making it an ideal option for scaling up to a pilot scale. Additionally, this system also has an integrated check weighing system that ensures that the filled capsules meet the required weight criteria, resulting in more accurate dosing. Dose accuracy and weight checks are crucial from both a quality and operator standpoint.
From a quality perspective, it is vital to ensure that each capsule contains the correct amount of medication. Even a small variation in dose can significantly impact the medication’s efficacy and safety. From an operator’s perspective, dose accuracy and weight checks can help to reduce the time spent in manufacturing. By ensuring that each capsule meets the required weight criteria, operators can avoid having to rework or reject capsules.
Historically, CDMOs have had to send powders and capsules to external labs for testing, which could be a time-consuming process, taking several days or even weeks to receive results. However, with Drum Lab, CDMOs can perform dose accuracy and weight checks in-house, significantly reducing the time required to develop and launch new products.
The Drum TT and Drum Lab are highly versatile systems that can fill capsules, as well as other customised containers or cartridges, for use in proprietary devices that may be in development. Both systems are capable of dosing blended and spray-dried powders with ease. The Drum TT and Drum Lab systems can be used in various scenarios, such as preparing small batches of a blended powder formulation for clinical trials or dosing spray-dried powder formulations for commercial production.
The Catalent facility also includes a Harro Höfliger Omnidose powder-filling machine, a versatile semi-automatic machine that can fill capsules with high precision. It is ideal for pharmaceutical companies working on DPI formulations, whether for new developments or scaling existing products. The Omnidose can be easily adjusted to handle different types of powders, making it an excellent tool for prototyping and developing new DPIs, while ensuring accurate powder filling in each capsule, which is crucial for the safety and efficacy of DPIs.
Furthermore, the design of the machine is scalable to meet the production-level demands of pharmaceutical companies. For larger quantities of powders, the Harro Höfliger Modu-C MS high-speed filling machine is capable of manufacturing up to100,000 capsules per hour. This advanced equipment can encapsulate a wide range of powders at kilogram scale, is capable of filling doses as low as 0.5 mg and is particularly useful for highly cohesive products, such as spray-dried formulations. The Modu-C MS also includes the inline capsule de-duster, 100% net weighing via a capacitive advanced mass measurement sensor for minimal quantities dosing and containment systems. The high-speed and high-volume capabilities of Modu-C MS can provide an efficient pathway to commercial production.
INTEGRATED SCALE-UP PATH
Developing sDPIs is a complex process that demands careful consideration and execution. One of the significant challenges in the process is scaling up capsule filling for clinical trials, which requires comprehensive planning and execution. During different phases of clinical trials, the number of capsules needed may vary significantly. It has been proven that scaling up drum filling is feasible, and the sDPI development process can be easily scaled up from Drum TT, Drum Lab and Omnidose to Modu-C MS. The Modu-C MS is designed to be compatible with the same dosing and filling systems used on the Omnidose, ensuring that the scale-up process is as seamless as possible. This makes it quick and simple to transfer the existing process parameters. This integrated scale-up path can help pharmaceutical companies accelerate the development and launch of new sDPI products while maintaining the highest quality and consistency at all production scales (Figure 2).
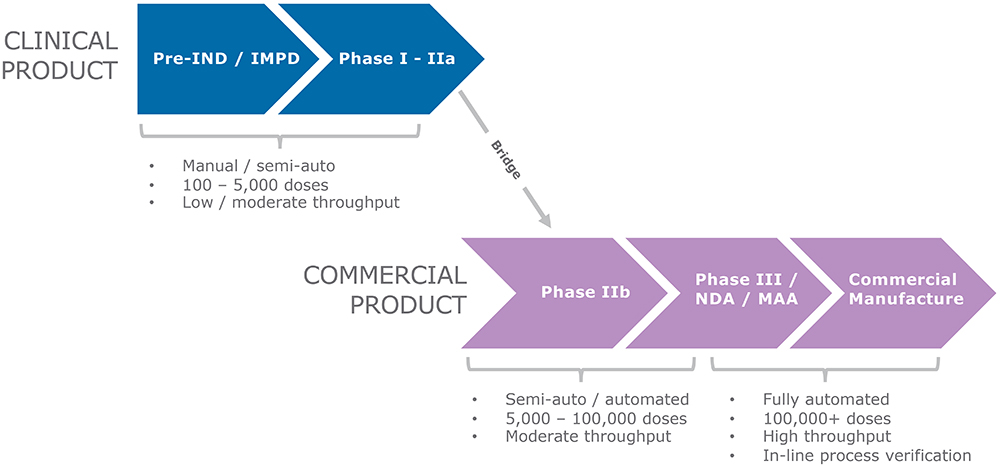
Figure 2: A seamless scale-up path for the development and manufacturing of an sDPI.
CONCLUSION
The development and manufacture of sDPIs present a multifaceted challenge that spans material selection, device design, powder formulation and filling technologies. Drum filling emerges as a superior method for capsule filling, offering high accuracy and speed, particularly beneficial for high-dose formulations. Catalent’s advanced dosing systems, including the Drum TT, Drum Lab, Omnidose and Modu-C MS, provide an integrated and scalable solution for sDPI development, from early-stage clinical trials to commercial production. To overcome the challenges associated with scaling up capsule filling, pharmaceutical companies can partner with a CDMO, such as Catalent, that has the necessary equipment and expertise to manage complexities, ensure quality and navigate regulatory requirements. By gaining access to this specialised knowledge and experience, pharmaceutical companies can de-risk the scale-up process and maximise the chances of commercial success. Partnering with a CDMO can mitigate the risks associated with scaling up and expedite time to market, ensuring both quality and commercial viability.
REFERENCES
- Pharmaprojects data. Citeline, data accessed Sep 2021.
- Son Y-J, Miller DP, Weers JG, “Optimizing Spray-Dried Porous Particles for High Dose Delivery with a Portable Dry Powder Inhaler”. Pharmaceutics, 2021, Vol 13(9), Article 1528.
- Ding L et al, “A Quality by Design Framework for Capsule-Based Dry Powder Inhalers”. Pharmaceutics, 2021, Vol 13(8), Article 1213.
- Buttini F et al, “Understanding the Importance of Capsules in Dry Powder Inhalers”. Pharmaceutics, 2021, Vol 13(11), Article 1936.
- Scherließ R et al, “Particle engineering in dry powders for inhalation”. Eur J Pharm Sci, 2022, Vol 172, Article 106158.
- Seyfang K et al, “Correlation Between Properties of Dry Powder Inhaler Model Formulations and Their Filling Performance: Comparison of Different Dosing Methods”. Proc RDD 2014, Vol 2, pp 427–432.
- Sibum I et al, “Automated Filling Equipment Allows Increase in the Maximum Dose to Be Filled in the Cyclops® High Dose Dry Powder Inhalation Device While Maintaining Dispersibility”. Pharmaceutics, 2020, Vol 12(7), Article 645.
- Jüptner A, Scherließ R, “Spray Dried Formulations for Inhalation- Meaningful Characterisation of Powder Properties”. Pharmaceutics, 2019, Vol 12(1), Article 14.
- Penachio ED, LaVigne K, “Dosator apparatus for filling a capsule with dry powder”. Patent US9642812B2, 2017.
- Maltz DS, Paboojian SJ, “Device Engineering Insights into TOBI Podhaler: A Development Case Study of High Efficiency Powder Delivery to Cystic Fibrosis Patients”. Proc RDD Europe 2011, Vol 1, pp 55–66.

