Citation: Marie C, Price T, Storz J, “Successfully Balancing the Needs of the Planet With the Needs of the Patient”. ONdrugDelivery, Issue 126 (Oct/Nov 2021), pp 46–50.
Christophe Marie, Taylor Price and Julien Storz chart the commitments and progress made to date by Aptar in achieving its sustainability targets. The authors also provide examples of integrated, circular economy initiatives undertaken by Aptar Pharma at a product solution level and comment on concrete commitments that can be made to further progress towards sustainability.
NO ONE IS ALONE IN THE JOURNEY TOWARDS GREATER SUSTAINABILITY
“To enhance understanding of how products could impact the environment, Aptar has developed an Eco-Design Tool that incorporates LCA functionality.”
Industry continues to transition away from the traditional, linear model of “take-make-consume-throw away” and adopt the values of the circular economy, where waste and pollution are designed out of product lifecycles. Furthermore, business leaders are increasingly aware of their responsibility to define their company’s own sustainability-related commitments and targets, and to ensure strategies are backed by authentic, measurable action. As such, with the sustainability credentials of the drug delivery sector coming under increasing scrutiny, learning from the experiences of other organisations or parent companies is one way to achieve this goal.
Aptar’s sustainability strategy considers the impact of its business from the perspective of people, product and the planet (Figure 1). Within that, the circular economy concept stands alone, defined by a self-contained vision and specific goals and targets, even though the principles of the circular economy touch all aspects of the business. Therefore, a key goal for Aptar is to show leadership through the implementation of innovative measures that will increase recycled content within products while improving recyclability, re-usability and compostability while also phasing out substances of high concern. The ultimate goal is to significantly reduce the volume of plastic ending up as waste. Collaboration with expert sustainability partners plays a crucial role in achieving this goal, ensuring that the company can improve upon what it understands and measures.
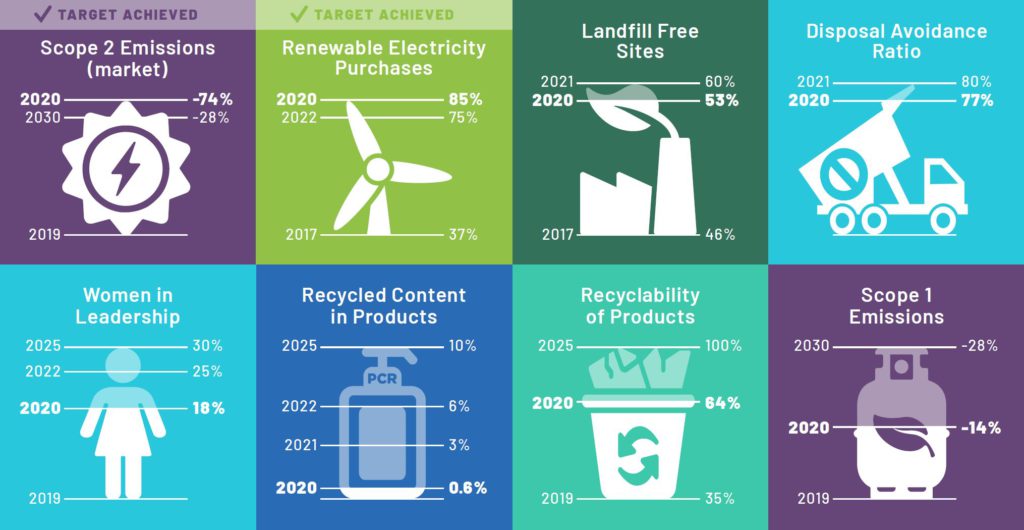
Figure 1: Aptar Group’s public sustainability commitments, designed to support the wider circular economy initiative.
One such partnership is Aptar’s involvement with the Ellen MacArthur Foundation (Cowes, UK), a non-profit organisation with a mission to accelerate the transition to a circular economy. Since 2019, Aptar has been an active member of the foundation’s “New Plastics Economy Global Commitment”. With their guidance, Aptar has led a recyclability work group with the foundation’s CE100 Network and piloted Circulytics, a tool which helps companies assess their circularity.1
“A key focus for any sustainability commitment in the pharmaceutical sector must be the identification and implementation of resins that can reduce that environmental impact.”
Aptar’s work with the Ellen MacArthur Foundation is complemented by its involvement with other global organisations focused on driving the circular economy. This includes membership of the World Business Council for Sustainable Development (WBCSD) (Geneva, Switzerland), through which Aptar has contributed to the quantitative, universal and transparent circular transition indicators (CTI) framework, designed to measure circularity and create a common language for all stakeholders. Aptar has also participated in the piloting of a Social Organisational Lifecycle Assessment (SO-LCA) tool with the United Nations environment programme.
The wider Aptar Group has expertise in the food, beverage, beauty, personal care, home care and active packaging sectors. As such, Aptar Pharma is able to benefit from considerable company-wide learning opportunities and initiatives, including cross-organisational collaborations that have sped up development times and derisked projects. More specifically, the highly regulated nature of the pharmaceutical market in which Aptar Pharma operates means that introducing ever more sustainability measures can only be achieved in line with the stringent parameters designed to guarantee product safety and efficacy, and to safeguard patient welfare. The following case studies highlight how Aptar Pharma continues to push its sustainability agenda forward within the limits of this framework.
ECO-DESIGN AND PRODUCT LIFECYCLE ASSESSMENTS
To enhance understanding of how products could impact the environment, Aptar has developed an Eco-Design Tool that incorporates Lifecycle Assessment (LCA) functionalities. Further improved in 2020 by adding additional enhancements, the company’s newly optimised LCA tools help product designers evaluate the inputs, outputs and potential environmental impacts of an entire product system throughout its lifecycle (according to ISO 14040) – from raw-material extraction through processing, manufacturing, distribution, use, re-use and maintenance through to disposal or recycling.
The key performance indicators measured by the LCA include CO2 footprint and recyclability, with Aptar’s development processes now including use of the LCA tool for all new Aptar Pharma product development projects.
This centrally developed, integrated initiative is an example of the company’s commitment to deliver holistic, enterprise-wide improvements, rather than isolated and fragmented initiatives. This approach to continuous cross-functional improvement has enabled advancements in several product areas.
AIRLESS+ DERMAL DELIVERY – ACCEPTED, ACCESSIBLE, AVAILABLE AND RECYCLABLE
The focus on sustainable design embodied in the Eco-Design Tool, has helped lead to important product breakthroughs at Aptar. An example is Aptar Pharma’s Airless+ range of highly recyclable products for dermal drug delivery, which addresses the need for greater patient protection, as outlined in the new US Pharmacopeia (USP) <661> “Plastic Packaging Systems and their Materials of Construction”, by using medical-grade resins.

Figure 2: (A) Aptar Pharma’s Airless+ product range, manufactured according to ISCC PLUS standards, has an excellent recyclability (cyclos-HTP AAA). (B) Aptar Pharma’s BOV products with cyclos-HTP certification grade A.
From a sustainability perspective, the Airless+ packaging ensures low amounts of residual product thanks to its high evacuation rate, which in turn supports product longevity. Additionally, as the range could be processed in existing recycling streams, it meets cyclos-HTP’s (Aachen, Germany) certification requirements – Airless+ has a rating of “Class AAA”, with a 96–98% “excellent recyclability” rate (for raw, natural packaging without décor and label) due to using only moulded components and no metal parts. More information is available directly from Aptar Pharma with respect to specific information regarding the regional application of the cyclos-HTP certification.
Airless+ is processed in existing recycling streams and is manufactured in a facility that has achieved ISO 14001 and ISO 50001 certifications (Figure 2A). This facility has also achieved the International Sustainability Carbon Certification (ISCC) PLUS, which is only awarded by employing a thorough internal traceability process along the entire supply chain, taking a “mass balance approach” to trace the flow of materials that are being mixed during production. This constant monitoring and counting approach makes it possible to trace the level and characteristics of circular and/or renewable content in the final product.
BAG-ON-VALVE – RECYCLABLE CONTINUOUS DISPENSING SOLUTION
Bag-on-Valve (BOV) continuous dispensing technology is another packaging application where patient care and the circular economy can both be achieved in equal measure. Widely recognised to deliver a cleaner, superior application through complete separation of product from propellant, Aptar Pharma’s BOV technology is also recyclable, as with the Airless+ range, achieving the cyclos-HTP qualification for “good recyclability” of the raw packaging (Figure 2B); specifically the certification was approved for Aptar Pharma’s BOV 30 mL, Pacifica Actuator and Aptar Pharma’s BOV 400 mL, Nasal EP Actuator including the cap and a standard aluminium can (more information is available directly from Aptar Pharma).
THE FUTURE LOOKS BRIGHT FOR FULLY RECYCLABLE MONO-MATERIAL PUMPS AND TUBES

Figure 3: Proventu, Aptar Pharma’s first mono-material tube.
Mono-material-based products are key in unlocking further gains in recyclability. In May 2021, Aptar announced the launch of the fully recyclable mono-material pump, Future, which has been designed using exclusively polyethylene (PE) material. Future is certified by cyclos-HTP and is graded class “A” by RecyClass (Brussels, Belgium) – a cross-industry initiative to establish traceability and a harmonised recycling approach in Europe.
Further cross-functional collaboration within Aptar Pharma includes the recent launch of Proventu, the company’s first mono-material tube for pharma (Figure 3). Using only polypropylene (PP), Proventu eliminates the need for a separate elastomer valve and incorporates a tethered cap, making it fully recyclable, while also preserving bulk integrity with a no-suck-back function. Proventu does not require any compromise from a user perspective, as it provides a no-mess, user-controlled application, one-handed closing and a dose measuring function. Proventu is also available with a child-resistant, senior-friendly (CRSF) option.
Aptar continues to develop innovative solutions that utilise mono-materials, having identified them as a key element of their recyclability strategy. Much like its commitment to source and using renewable feedstock, as outlined further in this article, Aptar sees this as a crucial aspect of its sustainability approach.
“When it comes to pulmonary drug delivery, decarbonisation efforts right now are dominated y a central challenge: how to move away from propellants such as HFAs HFA P227 and HFA P134a and towards newer, lower impact approaches.”
CREATING A SUSTAINABLE ALTERNATIVE MATERIAL AT SOURCE
A key focus for any sustainability commitment in the pharmaceutical sector must be the identification and implementation of resins that can reduce that impact. Right now, there are three available sustainable options for resins.
Mechanically recycled post-consumer resin (PCR) is a plastic sourced from waste that has been mechanically reprocessed for use in the manufacturing of new products. Although Aptar’s other businesses already use this material, mechanically recycled PCR is not yet able to meet all the regulatory and qualitative requirements needed for pharmaceutical use.
Chemically recycled PCR originates from plastic waste that cannot be recycled mechanically, such as coloured, multilayered or multi-material packaging and films. These materials can be recycled into a pharma-grade material that fulfils regulatory requirements, but is currently only available in limited quantities. However, the sustainability benefits are compelling; the process mitigates against fossil fuel depletion, contributes to waste management and reduces the CO2 emissions associated with incineration. As momentum continues to build behind the chemical recycling process, it is anticipated that higher volumes will be available as early as 2025.
The third option is the use of resins and chemicals from renewable feedstock, such as residue oils, fats and sustainably produced vegetable oils. As with chemically recycled options, pharma-grade polymers and chemicals can be achieved without impacting fossil fuel depletion; indeed, the process limits CO2 emissions in the atmosphere, contributing to a more circular economy. Both chemically recycled material and resin from renewable feedstock are managed through a mass balance accounting system and require a specific certification scheme, such as ISCC PLUS.
With a supply chain established and feedstocks demonstrating compatability with the sector’s specific regulatory requirements, these renewable resins will continue to emerge as a more sustainable option for pharma partners.
SHAKING UP THE pMDI INDUSTRY WITH A SUSTAINABLE ALTERNATIVE PROPELLANT
While developments such as this introduce greater potential for the sustainability of medical products in the future, when it comes to pulmonary drug delivery, decarbonisation efforts right now are dominated by a central challenge: how to move away from propellants such as hydrofluoroalkanes (HFAs) HFA P227 and HFA P134a and towards newer, lower impact approaches. Crucially, this challenge must be overcome without losing sight of two fundamental priorities: ensuring patient safety and supporting regimen adherence. The restriction on F-gases, including hydrofluorocarbons (HFCs) and HFAs used as propellants in pressurised metered dose inhalers (pMDIs), has been made official through the Kigali Amendment (2016) to the Montreal Protocol, which seeks to phase down the use of HFC/HFAs by 85% by 2047 across most countries.
Many drug delivery and pharma companies, including Aptar Pharma, are committed to defining the next phase of the pMDI market. HFA P152a and HFO1234ze are two potential alternative low global warming potential (GWP) propellants with compelling environmental cases. HFA P152a is entering its final year of an exhaustive full-inhalation propellant toxicology study and has raised no adverse findings so far. Although there are still important safety hurdles to be overcome before HFA P152a can be introduced as part of a marketable product, the current outlook is promising from a safety perspective.
It is promising from a sustainability angle too, with research presented by the University of Manchester (UK) showing that replacing HFA P134a with HFA P152a would reduce the climate change and global warming impacts of inhalers in the UK by 90–92%.2 HFO1234ze provides an even lower global warming potential (approximately a 99% reduction), as well as having a lower flammability concern. However, the long-term toxicology profile of HFO1234ze remains uncertain, with limited data available within the public domain to date. Aptar Pharma is working with both propellants with various collaborative partners, with both propellants offering exciting alternative pathways from a formulation stability perspective and a sustainability perspective.
A CIRCULAR ECONOMY REQUIRES SUSTAINABLE EFFORT AND INVESTMENT
The journey towards a circular economy has only just begun and there remains much work to do. The drug delivery sector has a real opportunity to make a meaningful and sustainable contribution and, to that end, Aptar has committed to some very clear, measurable, science-based targets. Firstly, to reduce absolute Scope 1 and 2 greenhouse gas emissions (GHGs) by 28% by 2030 and to reduce absolute Scope 3 GHG emissions by 14% by 2030. Secondly, by the end of 2021, more than 60% of Aptar’s manufacturing locations will be Landfill-Free certified through the company’s internal certification programme, and Aptar will achieve at least 80% disposal avoidance of operational waste globally. Thirdly, Aptar will continue to work towards the target of 100% renewable energy usage, having already surpassed its original 2022 goal.
FOR TODAY, FOR TOMORROW, FOREVER
As the famous Chinese proverb states, “A journey of a thousand miles begins with but a single step”. Aptar Pharma, as a forward-looking provider of drug delivery solutions, embraces its obligation to innovate in a way that successfully delivers on the needs of the patient while minimising the impact on the planet. As an individual company and as an industry, it is imperative to take the initiative, make those first steps and implement the measures that will introduce the benefits of the circular economy.
This is not a journey any organisation has to take alone. Access to resources, precedents and collaborative partners is greater than it has ever been. Methods for benchmarking, continuous improvement and measurement are also well established.
For Aptar Pharma, the benefit of forming part of a sustainably focused group has allowed and enabled some innovations to be delivered quickly, and without risk, to the benefit of the company’s pharma partners, patients and the planet. Other innovations are close to completion, while some remain in their infancy. Whatever the stage of development, it is important to keep in mind the ultimate goal – addressing social and environmental imperatives that create purpose and shared societal value, so that future generations may benefit from Aptar’s work.
It is Aptar’s deep commitment to create solutions that respect the environment, conserve natural resources and improve life on earth. As a leader in the drug delivery industry, it recognises, embraces and is determined to continue to lead the way in creating a circular economy, with repeatable and positive effects on people, the planet and products.
REFERENCES
- “Circulytics”. Ellen MacArthur Foundation website, accessed Oct 2021.
- Jeswani H, Azapagic A, “Life Cycle Environmental Impacts of Inhalers”. J Clean Prod, Nov 2019, Vol 237, Article 117733.
Previous article
SIX INHALER SUSTAINABILITY MYTHS – AND WHY THEY MUST BE BUSTEDNext article
ROUNDTABLE: ALLIANCE TO ZERO
