To Issue 127
Citation: Burnett H, Baron C, Bhogaita J, “Towards a More Sustainable Future with pMDI Solutions”. ONdrugDelivery, Issue 127 (Nov 2021), pp 26–30.
Howard Burnett, Chris Baron and Jay Bhogaita summarise the findings of a recent forum that looked at the development of more sustainable pressurised metered dose inhalers.
Those working in pulmonary drug and device development recognise the complexities associated with developing a combination product. For over 60 years, the challenges faced in delivering a pressurised metered dose inhaler (pMDI) to market have been largely consistent. These include ensuring valve compatibility with the drug formulation, safeguarding the integrity of the container closure system and adding in more measures to encourage greater levels of adherence – all while protecting both the efficacy of the drug product and the safety of the patient.
“For patients who currently prefer the familiarity and convenience of aerosolised inhalers, device choices in the future will increasingly be influenced by questions around sustainability.”
Perhaps one of the greatest challenges to date came in 1987 with the signing of the Montreal Protocol, and the initiation of phasing out chlorofluorocarbon (CFC) propellants. In 2016, the Kigali Amendment to the Montreal Protocol recognised that the hydrofluorocarbons (HFCs) that replaced CFCs are also powerful greenhouse gases with high global warming potential (GWP).1 Ratified by over 120 countries, this amendment brought in measures to phase down (rather than phase out) the production and consumption of 18 designated HFCs by more than 80% over the next 30 years.
And so, today, we are faced with a similar challenge to the phasing out of CFCs – how to move away from HFA propellants, such as 134a and 227, to an even more sustainable approach that further reduces the sector’s carbon impact.
As a market leader in respiratory devices, Aptar Pharma, together with Pharmaserve NW, recently convened a forum of experts to discuss “where next?” with the end-to-end development of more sustainable pMDI solutions. Recognising that only an integrated, collaborative approach involving experts throughout the product lifecycle can address the challenges ahead, Aptar Pharma and Pharmaserve NW invited a broad range of representatives to the forum, who collectively provided a high-level blend of scientific, pharmaceutical, clinical and engineering expertise. This article summarises the key findings.
“Given that there is mounting pressure from prescribers to find other, more sustainable alternatives, the future for pMDIs could be deemed to be up in the air.”
A MORE SUSTAINABLE FUTURE FOR pMDIS IS CRITICAL FOR WORLD HEALTH
Respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), are amongst the leading causes of death and disability worldwide. In the UK alone, the number of people to have received an asthma diagnosis is estimated to stand at 5.4 million2 – and COPD affects approximately three million people.3 Inhaled treatments, using devices such as pMDIs, dry powder inhalers (DPIs), soft mist inhalers (SMIs) and nebulisers, are used across the world as the mainstay of treatment for patients with respiratory conditions.
However, the healthcare market’s total contribution to worldwide net emissions (4.4% in 2014)4 continues to be a key focus for global and regional legislation, national healthcare guidelines, policies and recommendations. In this context, the use of HFCs in pMDIs forms a relatively small part of the greater whole. In the UK, for example, where pMDI use is proportionately higher than other European nations, it accounts for 3% of the UK NHS’s carbon footprint and 0.1% of the total national carbon footprint.5 Nevertheless, it remains imperative to reduce this figure further as part of a more sustainable future for pMDIs and other inhalation devices. It is also worth stating that, with the use of lower-GWP propellants, the contribution to carbon footprint may be lower or at least equivalent to other formats such as DPIs (Figure 1).
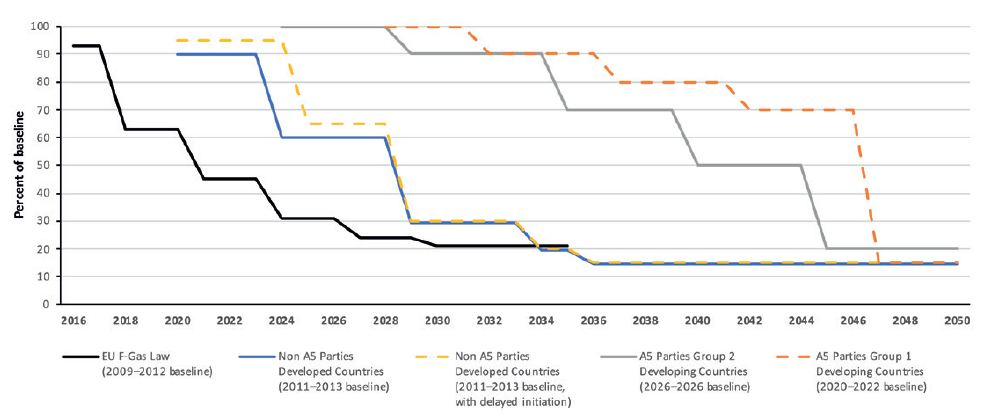
Figure 1: HFC phase-down schedules.6
FINDING THE RIGHT DRUG FOR THE RIGHT PATIENT AT THE RIGHT COST
From the forum’s clinician contributor, the message was clear: the climate crisis presents a considerable threat to human health and urgent, concerted action is required. Although the contribution of propellant-based pMDIs is relatively low in terms of their overall global carbon footprint, the transition to propellants with an even lower GWP will play an important part in improving the overall environmental burden associated with pMDI devices.
For patients who currently prefer the familiarity and convenience of aerosolised inhalers, device choices in the future will increasingly be influenced by questions around sustainability. As personalised medication becomes more prevalent, devices must therefore become much more fit for purpose, addressing financial burden, environmental cost, patient compliance and efficacy of treatment in equal measure.
IS THE FUTURE OF pMDIS UP IN THE AIR?
Given that there is mounting pressure from prescribers to find other, more sustainable alternatives, the future for pMDIs could be deemed to be up in the air. Taking the UK as an example, the NHS has set a target of a 50% reduction in the carbon footprint of inhalers by 2028. However, most DPIs and SMIs feature a large amount of plastic and/or metal components that will contribute to their GWP potential.
That said, a key consideration for the industry is the need to balance the cost of such devices, in terms of their impact on the environment, with the financial burden for payers. Delivering affordable reliever medication in a pMDI format will likely become more of a challenge than meeting emission targets, given that the decreasing use of current HFC propellants (p134a and p227) in other applications could lead to increased cost pressure on pMDI manufacturing. The year 2025 could see the tipping point, when there are no exceptions to reduction under the Montreal Protocol. It is possible that this could result in an increase in the price of medical-grade propellants, leading to a rise in overall manufacturing costs.
One of the benefits of the forum was the richness and diversity of viewpoints and data sources. While some experts maintain the world cannot afford not to switch as soon as possible, others express a more cautious tone. The forum was presented with a survey of 452 asthma and COPD patients who ranked “environmentally friendly” as the ninth most important characteristic of an inhaler.7 A further combined study of 150 asthma and COPD patients, 90 healthcare professionals and 10 NHS managers ranked “environmental impact” as an important factor in treatment decisions in 60%, 40% and 25% of respondents, respectively. Above all, cost was ranked as more important than any other consideration.
LEARNING FROM THE PAST TO DELIVER FOR THE FUTURE
One manufacturer of HFA medical propellants was clear that regulation will happen as the societal requirement for low-carbon inhalers (or low-GWP products) accelerates. Pharma and drug delivery supply chains need to react to find better products for partners and patients while learning the lessons from the CFC transition in the 1990s.
One of the challenges associated with the CFC to HFA transition was that the Montreal Protocol was implemented before 134a was fully proven for pMDI use. Indeed, 134a was, in part, selected because it was what chemical manufacturers had already identified as “their CFC-12 replacement”. The industry was not ready, so urgent technical, stability, formulation, materials and facility investment work was needed to meet the regulation within the timescales. This time we know it is coming and we need to be better prepared.
While low GWP is a key component, it should not be the only consideration in the development of a new propellant, it was argued at the forum. Stakeholder satisfaction is another key criterion. For pharma partners, the formulation must be optimal in both suspension and solution forms. It must be a similar cost to current propellants and the supply chain must be stable, with multiple options for supply. And, of course, the formulation must be safe for patients and meet the anticipated regulatory demand. From a lifecycle perspective, the propellant must display stable characteristics and not decompose when exposed to persistent environmental factors. And, in today’s world, recover, reclaim and re-use are core imperatives.
This transition should also be viewed as a generational opportunity to make improvements. The baseline is that the new propellant must be at least as good as 134a or 227 on pMDI performance measures. However, further investment, both financial and intellectual, is required to deliver the aspirational 90–99% environmental impact reduction and enable a more comprehensive coverage of available technologies – particularly in low-income economies.
“It is estimated that more than half a million tonnes of CO2 equivalent would be saved if every inhaler user in the UK returned all their inhalers for one year.”
SUSTAINABILITY IS PROPELLING IMPROVEMENTS IN CANISTER MANUFACTURE AND FILLING
While much of the focus is on discovering low-GWP propellants, there are notable advances coming from the wider supply chain, including canister manufacturers and filling lines. One expert at the forum presented the business case for the use of fluorocarbon polymerisation plasma, which produces a low surface energy (hydrophobic) fluorocarbon nanolayer on the internal surface of the cannister. Covalently bonded to the internal surface of the canister, this approach presents many benefits over the traditional solvent-based fluoropolymer-coated canisters, and notably less CO2 emissions, in fact 143 kg per million plasma-coated cans compared with 1,445 kg per million for anodised cans. Of course, formulations that only require uncoated canisters would not be subject to such a process and, as such, would further lower the GWP burden.
In terms of filling, there are clear opportunities to reduce CO2 emissions during the filling process, making it an important consideration for the development of low-carbon inhalers. Purging the can of air prior to crimping using propellant is a well-recognised process designed to remove any impurities, negate pressure rise within the can and eradicate the opportunity for reactions with the drug substance. Both traditional methods of purging result in propellant loss which, when filling large batches, constitutes a great deal of wastage. To prevent such wastage, an alternative purging technique, vacuum crimping, is being advocated, as the process has zero propellant emissions.
The change in propellant will, of course, have implications for existing filling lines designed and approved for use with incumbent propellants. Consideration must be given to the specific properties of 1234ze and 152a in areas such as flammability, meaning some investment will need to be made in new equipment infrastructure for filling.
WITHOUT BIOEQUIVALENCE, THERE WILL BE NO FUTURE
Of course, without regulatory approval, there is no future. And, for an issue that affects the planet, it is essential to address the requirements of regulatory bodies across the globe. To secure approval in the US, for example, the US FDA states that it will need to see “supporting data relating to the proposed variation(s)” in terms of input material specifications, device specifications, manufacturing process changes and product performance. To achieve bioequivalence (BE), the following performance criteria must be met: single actuation content, aerodynamic size distribution, spray pattern, plume geometry, priming and repriming.8
A major barrier to the development and proof of these criteria is the cost and requirements for clinical endpoint BE studies. Patient numbers can be significant, sometimes larger than the originator’s efficacy study, and considering the high variability, the low sensitivity and the inability to detect formulation differences, these studies are only confirmatory of local equivalence. One alternative is the SmartTrack™ integrated solution from Nanopharm, an Aptar Pharma company. This solution combines integrated device and formulation approaches, realistic performance testing tools and in silico modelling and simulation to streamline the project, reducing the time, cost and risk in demonstrating local BE (Figure 2).
Figure 2: SmartTrackTM – the unique, integrated solution to fast track local BE.
THE GRASS MAY NOT BE GREENER WHEN IT COMES TO REGULATORY APPROVAL
For a new chemical entity (NCE), the regulatory pathway for the development of pMDI products using newer propellants with lower GWP is clear – i.e. marketing authorisation application/new drug application (NDA). Clinical safety of new propellants would be evaluated in parallel to the NCE.
The replacement/repurposing of HFA 134a/HFA 227 in already approved pMDI products with lower GWP propellants could therefore be conceivably considered as hybrid generic development. However, the propellant makes up a significant proportion of the formulation composition, and there is poor availability and acceptability of human safety data on new propellants. As a locally acting medication, it will be more challenging to demonstrate BE.
Regulators may therefore consider switching the regulatory burden for low-GWP propellants to one like new product/NDA development, even though the active ingredient already has an established safety/clinical profile. This approach will increase the regulatory agency expectations for preclinical assessments, clinical safety and efficacy assessment, the weight of the stability data package at time of submission and the demonstration of BE. All of which could result in a significant impact on cost and timelines without implementing solutions such as SmartTrack™.
Clearly switching from HFA 134a/HFA 227 to lower-GWP propellants presents a series of different, less familiar challenges compared with “generic” inhalation development, with impacts on both pharmaceutical and therapeutic performance. The guidance from the forum’s regulatory panellist was conclusive: engage early with appropriate regulatory authorities to better understand the requirements for product-specific preclinical studies; be clear about the expectations for the demonstration of pharmaceutical and therapeutic equivalence, and establish the requirements for product-specific clinical efficacy and safety studies. Only then can device companies refine their development strategy and realistically assign costs and timelines based on clear regulatory expectations.
EVERY COMPONENT SHOULD BE EVALUATED AND IMPROVED
Many industries are transitioning away from the traditional, linear model of “take-make-consume-throw away” and adopting the values of the circular economy, where waste and pollution are designed out of product lifecycles. Drug delivery is no different, and the effective recycling of pMDIs will come under closer scrutiny. But this does require effort from all stakeholders, not least patients. While a large proportion of inhalers are consigned to landfill every year, it is estimated that more than 0.5 million tonnes of CO2 equivalent would be saved if every inhaler user in the UK returned all their inhalers for one year.9
WITH EVERY NEW CHALLENGE COMES NEW OPPORTUNITY
It is overstating this situation to call it a crisis but it is certainly a time of uncertainty, promise and opportunity. In isolation, the transition from propellants such as hydro fluoroalkanes HFA 227 and HFA P134a towards newer, lower-impact approaches is a sizeable task. However, this is a once-in-a-generation opportunity – one that cannot be missed – to ensure a more sustainable future for pMDI solutions for those millions of patients who need or prefer it.
Truly grasping this opportunity means learning the lessons from the CFC to HFA transition, including the need for effective collaboration, to ensure all the various complex and interconnected elements are managed in unison through the prism of a robust multi-stakeholder partnership. At the same time, priority must continue to be given to patient care while also encouraging innovation and allowing sufficient time for research and development activities. By adopting these measures, the industry can again demonstrate its collective ability to rise to the urgent global challenge presented by climate change.
To learn more about Pharmaserve NW, please visit: www.pharmaservenorthwest.co.uk/technologies.
REFERENCES
- “The Kigali Amendment 2016: The amendment to the Montreal Protocol agreed by the Twenty-Eighth Meeting of the Parties”. Oct 10–15, 2016.
- “Asthma facts and statistics”. Asthma UK (https://www.asthma.org.uk/about/media/facts-and-statistics/).
- “Chronic obstructive pulmonary disease: How common is it?”. NICE, July 2021.
- “Health Care Without Harm: Health care climate footprint report”. Climate-smart health care series, Green Paper No 1, Sep 2019.
- “Minimising the environmental impact of inhaled therapies: problems with policy on low carbon inhalers”. Eur Respir J, 2020, Vol 55, 2000048.
- “Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on fluorinated greenhouse gases and repealing Regulation (EC) No 842/2006”. EU Policy Document.
- Kocks JW et al, “Inhaler preferences and satisfaction of asthma and COPD patients”. Conference Poster, Eur Resp Soc Congress, Paris (France), Sep 15-19, 2018. In Eur Resp J, 2018, Vol 52, Suppl 62, Poster Abstract 1009.
- “Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs – General Considerations”. US FDA, Mar 2014.
- “What to do with inhalers”. Web Page, Recycle Now, Accessed November 2021. (https://www.recyclenow.com/what-to-do-with/inhalers-0).

