To Issue 131
Citation: Baron C, Shur J, “Investigating the Propellant Pathways Leading to a Sustainable Future for MDIs”. ONdrugDelivery, Issue 131 (Apr 2022), pp 49–52.
Chris Baron and Jag Shur discuss the various dynamics involved in realising the full potential of MDIs to deliver both improved environmental performance and even better patient outcomes.
From the phones we use to the food we eat, every aspect of our daily lives carries with it an environmental cost. Through complex chains of production, distribution and consumption, modern living contributes to the generation of harmful emissions into the Earth’s atmosphere, which, in turn, are responsible for trapping heat and causing the greenhouse effect that is responsible for global warming.
Carbon dioxide (CO2) remains the leading greenhouse gas emitted through human activities. This is primarily a result of the burning of fossil fuels for transportation and energy but other activities, such as industrial processes and agricultural practices, are also contributing factors. As a result, global CO2 emissions have increased around tenfold over the past century. In a bid to slow global warming and reduce the impact of climate change, governments and policymakers around the world have set out targets to reduce emissions of CO2 and other greenhouse gases with global-warming potential (GWP), such as methane (CH4) and nitrous oxide (N2O).1
This list of gases with high GWP also includes synthetic fluorinated gases, or F-gases, which have a variety of applications, both industrial and medical. For example, F-gases are currently used as propellants in metered dose inhalers (MDIs), playing a vital role for millions of patients worldwide in the management of respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD).
While they are now the standard, F-gases were only introduced to MDIs in the 1990s as a replacement for the chlorofluorocarbons (CFCs) used previously. This change was driven by the fact that CFCs were found to have a depleting effect on the Earth’s protective ozone layer, weakening its ability to absorb ultraviolet radiation from the sun and protect against warming. As a result, on September 16, 1987, global powers set down an agreed timeline for the phasing out of CFCs by January 1996 within the United Nations Environment Programme (UNEP) treaty known as the Montreal Protocol.
“There is a clear need for pharmaceutical companies and their drug delivery solutions partners to facilitate a safe, speedy and smooth transition to alternative MDI propellants.”
Because of their role as an essential healthcare product, CFC-based MDIs were exempt from this deadline until suitable alternatives were made available. This challenge prompted a surge of innovation in the sector that resulted in the introduction of the hydrofluorocarbons HFA 134a and HFA 227a as CFC replacements. The first products to employ these F-gases were Airomir (Teva, Jerusalem, Israel), which launched in the UK in 1995, and Proventil (Merck & Co, NJ, US), which was introduced to patients in the US in 1996. Eventually, CFCs were confirmed as being fully phased out of MDIs in Europe in 2010 and in the US in 2011.
With respect to repairing the ozone layer, the Montreal Protocol has been an unquestionable success. Experts suggest the Antarctic ozone hole will close by the 2060s, while ozone at other affected regions is expected to return to pre-1980s levels even sooner.2 In addition, by moving away from CFC propellants, MDIs registered a reduction in CO2 emissions, with values for HFA-based inhalers between three and nine times lower than those registered by their CFC predecessors when compared on a like-for-like basis.
In the intervening years, however, the emergence of further information about the properties of F-gases has prompted the need for further change. While they might not deplete the ozone layer like CFCs and are emitted in much lower volumes than CO2, methane and nitrous oxide, F-gases have a comparatively higher GWP and, therefore, a proportionately higher impact on climate change.
Of course, efforts to limit the environmental impact of MDIs using F-gases must be understood within context. Healthcare, as a sector, is estimated to be responsible for 4–5% of worldwide greenhouse gas emissions, and that figure is driven by myriad factors.3 Focusing on the NHS in the UK, medicines – including inhalers – are responsible for 25% of its overall carbon emissions. However, the overwhelming majority of that figure (80%) relates to manufacturing and freight emissions in the supply chain.4 With inhalers responsible for 3% of the carbon footprint of the NHS, and 0.1% of the UK’s total national carbon footprint,5 a transition to lower GWP propellants will, therefore, ensure inhalation devices play their part in healthcare’s broader push towards net-zero goals.
In Europe, this issue has been tackled through the F-Gas Regulation, which came into force in January 2015, incorporating bans and quotas on the use of F-gases with an objective to phase down their use to one-fifth of 2014 sales levels by 2030.5 In the US, the Environmental Protection Agency is also driving the transition to F-gas alternatives through its Significant New Alternatives Policy and, more recently, via new regulation within the American Innovation and Manufacturing Act, which was enacted in December 2020. At an international level, the issue has been addressed through the 2016 Kigali Amendment to the Montreal Protocol, which commenced the phasing down of F-gases from January 2019 (Figure 1).
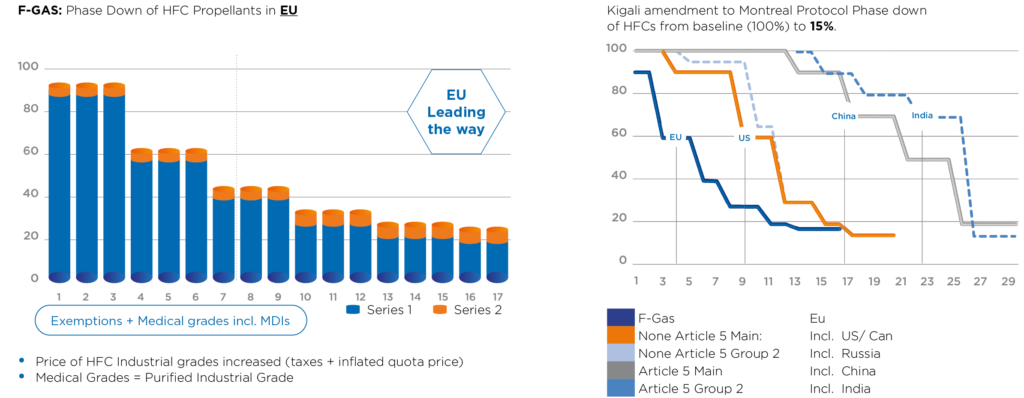
Figure 1: Regulation based on controlling emission of CO2 – tonnes equivalent.
Given that the majority of inhalers currently in use are MDIs using either HFA 134a or HFA 227a, and as volumes of MDI F-gas in the US are projected to increase from 1,491 metric tons in 2020 to 1,595 in 2025,6 there is a clear need for pharmaceutical companies and their drug delivery solutions partners to facilitate a safe, speedy and smooth transition to alternative MDI propellants.
This situation is further complicated by the likelihood of cost increases as sources of industrial-grade F-gas volumes decline. Indeed, significant rises have been registered in the price of HFA medical-grade propellant, in particular HFA 227a, throughout 2020 and 2021, which will impact the respective manufacturing costs for MDIs based on this propellant.
“Beyond technical performance, it is also important to consider how
MDIs are valued by patients.”
The transition to low-GWP propellants has been given further urgency by the environmental ambitions and sustainability targets being set out by major healthcare providers. For example, NHS Primary Care Networks in the UK has announced its intention to reward, where appropriate, the prescribing of lower carbon-footprint alternatives to MDIs, such as dry powder inhalers (DPIs) and soft mist inhalers (SMIs). This is in line with NHS England’s stated goal for just 25% of prescribed non-salbutamol inhalers to be MDIs by 2023/24.7,8 Future targets will also reward the prescribing of MDIs with a lower carbon footprint while also encouraging patients to return used inhalers to pharmacies for safe and environmentally friendly disposal.4
While framing the use of alternative device types as a straightforward switch might make the process sound simple, the reality is more complicated. Despite their specific advantages, DPIs and SMIs do not necessarily make for a true like-for-like choice, with MDIs offering significantly lower cost per dose in a device form that has been proven over many years as the dominant mechanism for the delivery of drugs targeting the respiratory system.
Beyond technical performance, it is also important to consider how MDIs are valued by patients. For millions of people, these devices are deeply embedded into their daily lives for maintenance medication, and they also know that they can rely on them to deliver rescue medication in times of emergency.
It is for these reasons that, in the respiratory drug delivery sector, sights are keenly trained on the successful transition to low-GWP propellants as current high-GWP variants are phased out. As with the transition away from CFCs, these new options must be assessed thoroughly using strict criteria, with robust evidence that they deliver continuity of both functional and pharmaceutical performance while answering concerns in the areas of toxicity, flammability and environmental impact.
Based on these criteria, the leading propellant options for the next generation of low-GWP MDIs are HFA 152a and HFO 1234ze from the hydrofluoroolefin family. Much work has already been carried out by industry stakeholders to evaluate these gases. But further work is still required to build a complete picture of their chemical and physical properties as propellants, as well as their suitability and safety within drug delivery systems.
Thinking specifically of performance within an MDI, key areas to consider for these propellants are the solubility of an API in the propellant system; the profile of the emitted spray, looking at droplet size and evaporation of the propellant; and the electrostatic charge properties, which will define how they are handled and their relationship to regional deposition in the airways.
Aptar Pharma takes a holistic approach to drug delivery to enable pharma partners to answer such questions by drawing on the specialist expertise within its broad operational portfolio. Analytical studies carried out within its Nanopharm business, for example, have provided rich data and insights to compare the characteristics and performance of existing propellants against low-GWP alternatives.
The solubility of a drug substance in low-GWP propellants is a critical consideration on this journey. This measurement illuminates the potential for the formulation to be developed as either a solution or suspension. In the case of suspension systems, solubility level will also determine the stability of the particle size. In the case of solutions, it will directly influence the amount of co-solvent required, which, in turn, impacts the velocity of the aerosol and the droplet-evaporation kinetics.
To explore the issue of API-propellant solubility, Nanopharm has used high-pressure nuclear magnetic resonance spectroscopy. In one such study looking at beclomethasone in low-GWP and existing propellants, the solubility of HFA 152a was found to be 400 μg/mL, which was more than twice the level observed with HFA 227a and three times the level observed with HFA 134a and HFO 1234ze. Such findings have important implications for formulation design, including the potential to lower the co-solvent load in ethanol-based formulations where the propellant solubility is observed to be higher.
Nanopharm’s work has also encompassed analysis of the aerosol process to measure variances in droplet size among the various propellants. This test environment uses phase doppler anemometry to measure the evolution of the droplet size issued from placebo canisters under no-flow conditions and also under flow rates of 30 L/min to approximate patient use.
Under no-flow conditions, the droplet size of HFO 1234ze aerosols decreased to a less significant degree before reaching stability. Under flow conditions, however, the droplet-size evolution of HFO 1234ze is more closely aligned with HFA 227a, demonstrating an initial decline in droplet size prior to a subsequent rise as distance increases. In the case of HFA 152a, meanwhile, the droplet-size evolution was observed as being similar to HFA 134a, with both propellants maintaining a small droplet size as they evolve.
Uncovering this level of understanding of droplet dynamics and the differences in aerosol velocity provides important insight into deposition behaviour and can be valuable in controlling aerosolisation through optimisation of actuator and valve design.
Along with electrostatic charge behaviour, the properties of these propellant aerosol systems are also likely to impact regional deposition in the airways. Nanopharm has collaborated with Dr Philip Kwok, Lecturer in Pharmaceutical Sciences in the School of Pharmacy at the University of Sydney (Australia), to investigate the aerosol charge of propellant systems using the Bipolar Charge Analyzer (BOLAR) system from Dekati (Kangasala, Finland). This collaborative research has been able to establish not just the net charge of propellants but charge polarity. Overall, the propellants demonstrated more electropositive charge. But it was notable that HFA 152a showed greatest charge propensity and HFO 1234ze showed the least, with near-neutral charges of both polarities – results that may be correlated to the previous findings on solubility.
Equipped with observed data on the behaviour of the propellants, Nanopharm has also investigated the fate of inhaled doses using its SmartTrack™ platform. This platform provides an assessment of aerosol properties under realistic conditions, facilitating an understanding of formulation microstructure using dissolution and spectroscopic methods, and employing physiologically based pharmacokinetic (PBPK) modelling to simulate regional deposition and systemic exposure.
In-house studies were carried out using SmartTrack™ to assess the differences in performance between a current Ventolin (salbutamol, GSK) inhaler and an approximated MDI containing salbutamol in combination with HFA 152a. Deposition analysis showed that the aerodynamic particle-size distribution was comparable in both cases, as was the predicted regional lung distribution.
A key difference, however, was the higher deposition levels in the throat and upper airways for the salbutamol/HFA 152a formulation, which led to a slightly lower total dose being emitted and lower deposition in the alveoli of the deep lung. This indicates the potential for variance in systemic effect while underlining the importance of the valve/actuator interaction in managing these variables (Figure 2).
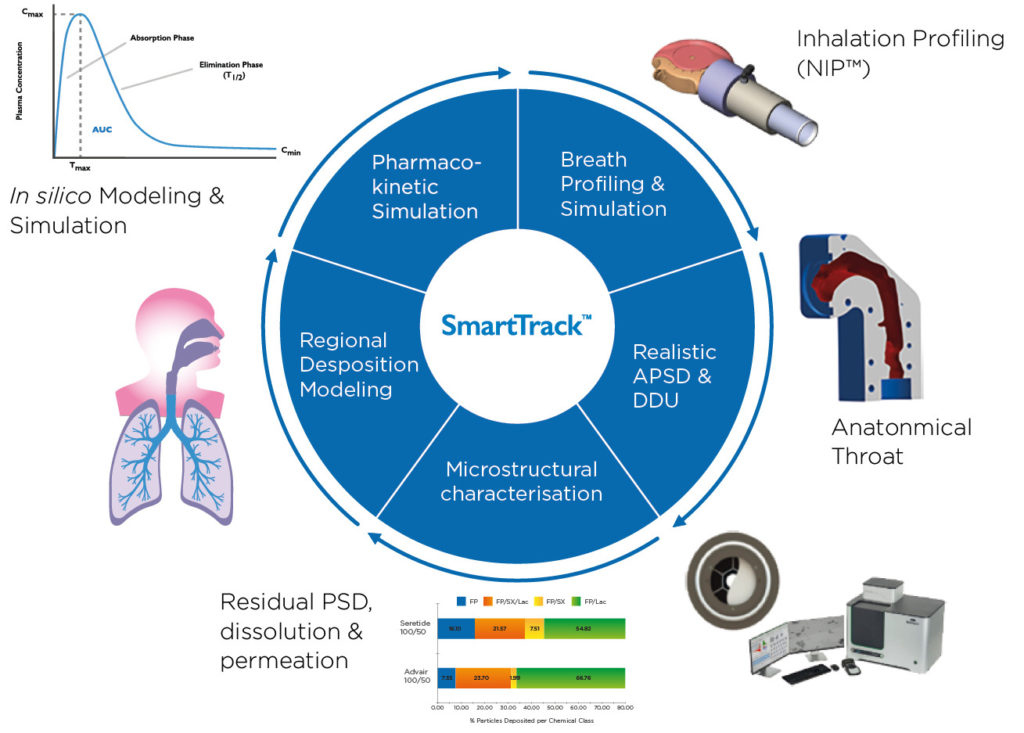
Figure 2: A combination of realistic in vitro testing and in silico modelling was used to compare the systemic exposure of salbutamol formulated into HFA 152a with Ventolin.
Taken together, the results from these tests provide a range of illuminating datasets on variances between propellants in areas including API solubility, particle aerosolisation, electrostatic charge, deposition and distribution, all of which are highly valuable when making key decisions around formulation and device development. To ensure pharma partners are fully supported in this area, Aptar Pharma has also invested in a pilot mixing-and-filling suite to handle all MDI propellants, including HFO 1234ze and HFA 152a, with the first customer filling having taken place in September 2021.
From analysis of formulation and propellant to device development, regulatory support and commercialisation, Aptar Pharma’s expanded suite of devices and harmonised service offerings are positioned to answer the increasingly complex questions regarding the low-GWP future of MDIs. Equipped with new metering valve technology featuring materials and geometry that are designed for optimal compatibility with formulations based on HFO 1234ze and HFA 152a, there is evidence of a clear development pathway between existing HFA-based MDIs and a new generation of MDIs using lower GWP alternatives.
Drawing on the learnings from the CFC-to-HFA transition, consistency will be an important factor in this journey, providing patients with the continuity of familiar, proven healthcare solutions that are, at the very least, comparable to existing solutions.
With the industry at an inflexion point, stakeholders in the sector have an opportunity to influence wider beneficial change beyond the transition to low-GWP propellants. Addressing levels of compliance and adherence among patients, for example, can help to tackle the estimated 250,000 tonnes of CO2 equivalent generated annually from overuse of short-acting β2-agonist (SABA) inhalers in the UK,9 while also avoiding medicine and device waste. Further possible measures include the use of primeless valves to minimise dose wastage, the inclusion of dose counters to encourage regimen adherence, the use of breath-activated inhalers to address co-ordination issues and improve patient compliance, and the integration of digital health platforms to further enhance patient engagement.
So, while we must focus on building our understanding of the properties of low-GWP alternative propellants and exploring all the new avenues they offer, it is also an opportune moment to zoom out and reflect on all the various dynamics surrounding formulation, device and delivery. It is only by taking this holistic approach that MDIs can truly realise their future potential in delivering both improved environmental performance and even better patient outcomes.
For more information about sustainable pMDIs, visit: www.aptar.com/resources/investigating-the-propellant-pathways-leading-to-a-sustainable-future-of-mdis
REFERENCES
- “Overview of Greenhouse Gases”. US Environmental Protection Agency.
- “Rebuilding the ozone layer: how the world came together for the ultimate repair job”. UN Environment Programme, Sep 15, 2021.
- “Health care’s response to climate change: a carbon footprint assessment of the NHS in England”. Lancet Planet Health, 2021, Vol 5(2), E84-E92.
- “Delivering a ‘Net Zero’ National Health Service”. UK NHS.
- “EU legislation to control F-gases”. European Commission.
- “Market Characterization of the U.S. Metered Dose Inhaler Industry”. ICF, Sep 2021.
- “MIMS introduces leaf symbol to support sustainable inhaler prescribing”. GP, Dec 8, 2021.
- “Primary care networks incentivised to switch patients to more environmentally friendly inhalers”. Pharm J, Sep 3, 2021. 9. “New data show overuse of reliever medication in asthma is responsible for 250,000 tonnes of greenhouse gas emissions every year in the UK”. AstraZeneca, Feb 17, 2021.

