To Issue 151
Citation: “Technology Showcase: CCBio’s Felice Dose – Simplifying Subcutaneous Infusion”. ONdrugDelivery, Issue 151 (Sep 2023), pp 70–71.
CCBio showcases Felice Dose, a revolutionary on-body injector (OBI) designed to address the challenges of traditional IV injections and SC drug administration.
Transitioning from intravenous (IV) to subcutaneous (SC) injections has several advantages – SC injections are more comfortable for patients during administration, more convenient, don’t require finding a suitable vein and can be self-administered, empowering patient autonomy. SC injections also reduce the requirement for hospital visits, achieve more stable drug absorption, allow for lower drug dosages and minimise side effects by reducing direct contact with blood and organs. Transitioning to SC administration offers a more comfortable, patient-centric experience, reducing healthcare resource use and improving drug delivery effectiveness. As such, SC administration emerges as the preferred drug delivery method in various scenarios.
“SC injections are more comfortable for patients during administration, more convenient, don’t require finding a suitable vein and can be self-administered, empowering patient autonomy.”
Felice Dose is a revolutionary on-body injector (OBI) designed to address the challenges of traditional IV injections and SC drug administration (Figure 1). The device aims to provide a more efficient and comfortable drug delivery method for patients and meet the evolving needs of the pharma and biotech industries.
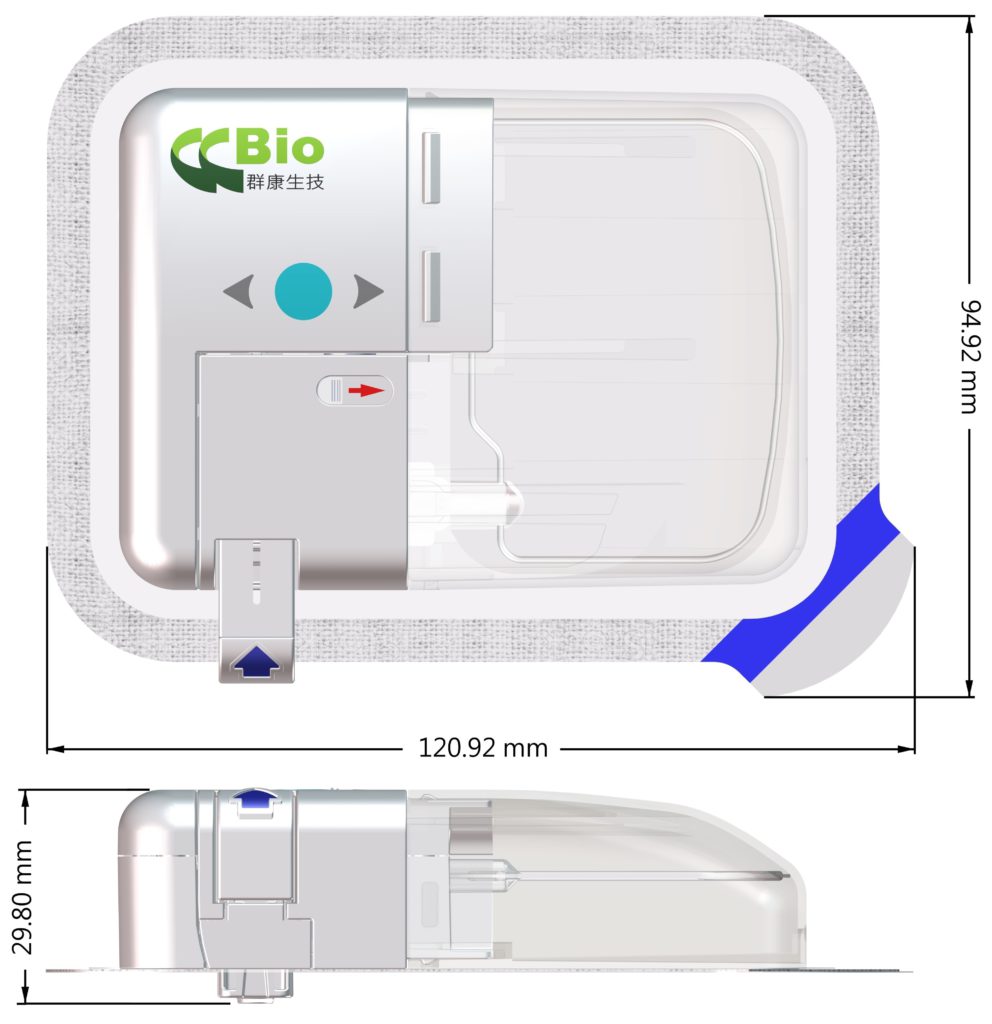
Figure 1: Felice Dose – CCBio’s on-body injector.
“Previously, patients have had to spend an hour or more in the hospital for SC infusions, but Felice Dose enables them to perform an SC infusion conveniently at home or work, or during travel.”
Felice Dose addresses the high failure rate of IV injections due to vein inflammation or phlebitis by offering an SC drug delivery device with a stable delivery motor system. This ensures a stable dose of the drug per unit time, reducing swelling and pain underneath the skin through a discontinuous pressure mechanism that prevents discomfort during administration (Figure 2).
Figure 2: Felice Dose delivers a stable dose of drug per unit time.
Figure 3: Felice Dose is capable of delivering large volumes, long-duration injections and varying drug viscosities.
One of the key advantages of Felice Dose is simplifying SC infusion. Previously, patients have had to spend an hour or more in the hospital for SC infusions, but Felice Dose enables them to perform an SC infusion conveniently at home or work, or during travel, while meeting safety and hygiene standards. This convenience improves patients’ quality of life, offering flexibility and reducing the burden of needing to visit a hospital or clinic.
Felice Dose is capable of handling large drug volumes, varied and long-duration injections and different drug viscosities (Figure 3). It offers flexibility in terms of primary drug container, needle gauge, needle length and user interface, allowing pharma to customise the drug delivery experience according to their patients’ specific needs and preferences.
Felice Dose uses a mini-bag elastic polymer container system, allowing dose volumes to be adjusted from 5 to 250 mL. The device also incorporates steel needles of varying gauges and soft needles to maximise patient convenience and minimise pain during skin penetration. Felice Dose empowers patients by giving them a greater sense of control and autonomy over their treatments. With medical guidance, patients can independently manage their treatment process, leading to improved treatment effectiveness and overall satisfaction.
The user interface of Felice Dose is well designed and technologically advanced, featuring options for WiFi, near field communication (NFC) and Bluetooth connectivity. The fully-customisable programmability empowers patients to take control of their treatment, making their daily lives easier and more comfortable. With this level of control and connectivity, the need for frequent hospital visits and healthcare provider involvement is reduced.
The use of a lithium battery and the delivery motor system’s capability to function without continuous power contribute to the device’s overall lightweight design, enhancing patient comfort during wear. This design also aligns with the industry’s increasing interest in SC drug formulations and wearable OBIs.
The Felice Dose device has undergone successful experiments, proving its capability to handle a 20 mL injection for up to one hour. In this experiment, CCBio used a standard viscosity solution without any drug. During the experiment, a flexible drug bag was filled with 21 mL of the non-drug standard viscosity solution. The drug bag was then placed on a microbalance with a precision of three decimal places to continuously record data, including the accumulation of the solution over time.
From the experimental results, it was observed that the accumulation of the solution during the injection process exhibited a linear slope, and the injection of the solution was completed within the predetermined standard time, leaving approximately 1 mL of residual solvent (Figure 4).
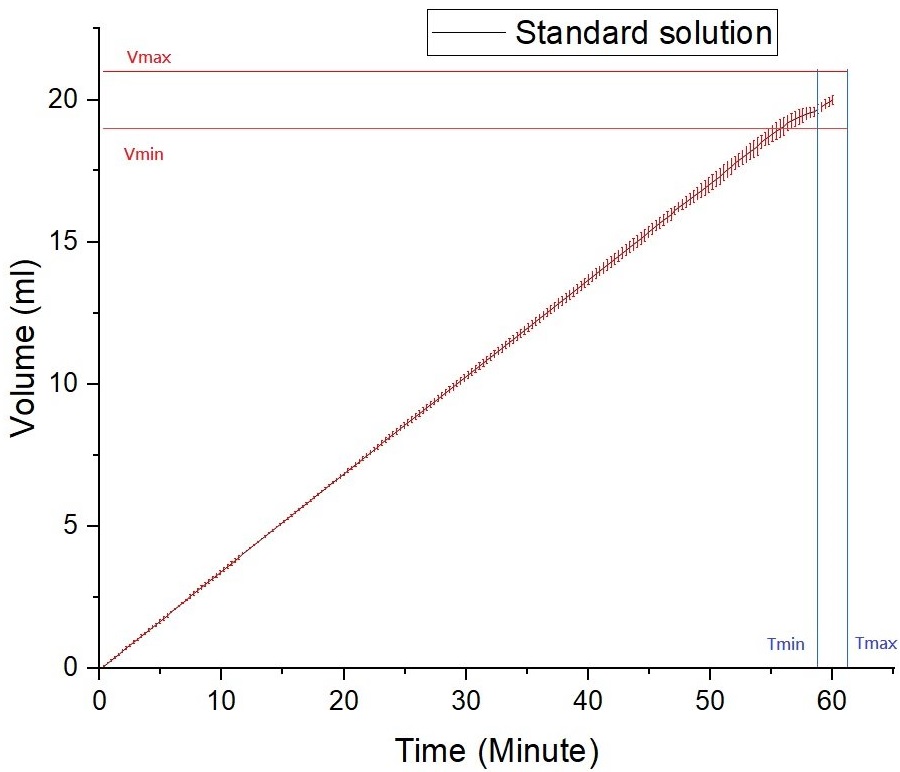
Figure 4: Experimental results from an injection of standard viscosity solution without drug using Felice Dose.
Find out more about Felice Dose here: www.ccbio.com.tw/prodview20.html.

