To Issue 138
Citation: “Technology Showcase: CCBio’s I-Platform – How to Upgrade a Device in One Platform”. ONdrugDelivery, Issue 138 (Oct 2022), pp 102–103.
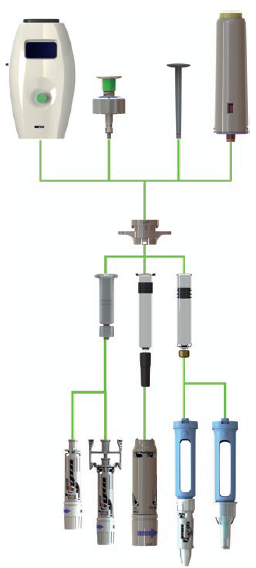
Figure 1: The I-Platform drug delivery device – showing the delivery units on the top, the containers and their adapters in the middle and the safety needles on the bottom.
In 2020 and 2021, the covid-19 pandemic had a dramatic impact on global healthcare systems. Lockdowns, limitations on mobility and concern about cross-contamination resulted in many patients with chronic diseases being unable to travel to hospitals and clinics to have their necessary medications administered. The pandemic also massively increased the workload for healthcare organisations and their staff, meaning they were often overwhelmed to the point that they struggled to serve their communities except for providing care to those in critical and urgent conditions.
“The I-Platform enables pharma companies to launch their drug initially with a simple, easy-to-use device configuration … then, later on in the product lifecycle, the device configuration can be further upgraded to an advanced model.”
This poses the question – is it possible for more patients to administer their medication at home? Historically, the injection process has involved withdrawing the drug from a vial into a syringe and then injecting into the human body. This is already a non-trivial process for a trained health provider to handle precisely and accurately, so expecting untrained users to perform their drug injections this way at home would introduce an unacceptable level of risk and danger.

Figure 2: The basic I-Platform PFS
with holder.
Originally, pen injectors and autoinjectors were considered the solution for enabling at-home self-administration. However, even a simple fixed-dose pen injector may take years and significant investment to develop. Additionally, as became starkly clear during covid-19, limitations on business travel and increasing transportation costs can noticeably hinder the development for a new device. As such, there is pressure on healthcare systems, pharma companies and device manufacturers to come up with a better, faster and more flexible development method so that patients and users can receive more injectable drugs at home. An additional consideration for pharma companies is the need to think about what kind of device is most suitable commercially; sometimes, the marketing evaluation will require additional time to find an alternative device to the initial choice.

Figure 3: An I-Platform delivery device with a safety device.
CCBio designed the I-Platform to solve this issue (Figure 1). The I-Platform is designed so that, at the start of development, the pharma or biotech company chooses a prefilled syringe (PFS) with holder for the initial stages, allowing them to save costs and time. During the early stage of Phase II studies, pharma can use the PFS, which enables users to perform injections easily, as the holder provides larger finger contact and a holding flange (Figure 2). CCBio designs different holders for major containers and can also fit safety devices for preventing needlestick injuries (Figure 3).
The delivery unit is the core technology of the I-Platform, which allows pharma companies to update the driver, such as a spring rod or an electric rod, during development (Figures 4 & 5), which is more convenient for assembling the PFS with the end device. Additionally, using a commercial device at this stage saves costs in the long run because, during Phase II and III, it enables the pharma company to upgrade the device from a simple, conventional delivery unit to a complex one quickly and easily.

Figure 4: An I-Platform delivery device with a spring rod.
The I-Platform enables pharma companies to launch their drug initially with a simple, easy-to-use device configuration that will reduce the burden on hospitals and healthcare facilities by enabling increased adoption of at-home drug administration. Then, later on in the product lifecycle, the device configuration can be further upgraded to an advanced model with much better functionality and performance.
However, the intended use of the I-Platform is not exclusively for home use. Tesla is the crown jewel of the I-Platform, with advanced and innovative features, including:
- WiFi connectivity
- Near-field communication connectivity
- LED display
- Bluetooth connectivity
- Fully customisable programming
- Powerful server motor
- Li-ion battery.
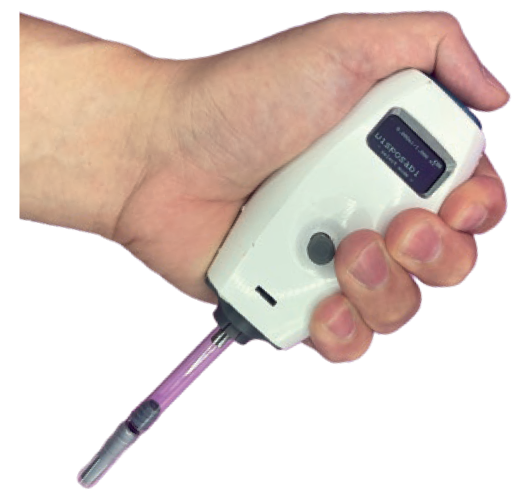
Figure 5: The I-Platform Tesla.
Tesla, with the I-Platform’s most advanced delivery unit, is capable of handling various drug viscosities, as well as injection speeds and durations. The smart program can help patients easily control their treatment, making their daily life easier, more comfortable and less dependent on visiting hospitals and healthcare providers, in addition to reducing the overall cost of their therapy.
The I-platform Tesla also has many new functions to distinguish new biologic or biosimilar drugs on the market. CCBio can design a unique and special holder construction to distinguish specific drugs, which will significantly contribute to preventing healthcare providers from delivering the wrong medication.
The I-Platform can not only be upgraded quickly, but also it follows environmental, social and governance principles. The I-Platform Tesla can be made with a reusable part so that the pharma company will only need to provide the drug without the plunger rod for repeat uses. This reduces the required logistics space, packaging costs and shipping costs. CCBio knows that product space efficiency is key, especially in a pandemic.
In recent years, there have been more and more viruses infecting humans. The covid-19 pandemic exposed the serious truth that we are unprepared and vulnerable before nature. As such, it is imperative that the pharmaceutical industry learns the lessons of the past few years and prepares for the future. It will be key to transition more drug delivery to the home as part of these preparations. Through a joint effort between pharma companies and medical device developers, CCBio believes that it is possible to overcome the pandemic and return to normal life again.

