Citation: Romańczuk R, Duband S, Chandra A, “The Added Value of a Platform Approach: Pen Injector Case Study”. ONdrugDelivery, Issue 120 (May 2021), pp 22–26.
Radosław Romańczuk, Severine Duband and Audrey Chandra discuss the development of the company’s pen-injector platforms and how they are tailored to meet the customer’s needs.
“In order to increase patients’ compliance and adherence, as well as to accommodate pharmaceuticals’ needs, developing a device platform needs to be strategically defined to ensure that it is designed for the maximum possible range of potential therapeutic areas and patient populations.”
INJECTION AND PATIENT ADHERENCE
Parenteral administration is preferred in emergencies related to cardiac diseases or anaphylactic shock, and, in certain therapies, it is the only possible route of administration (monoclonal antibodies (mAbs), insulin, etc). In comparison with oral administration, injection has a number of significant advantages, such as better bioavailability, faster onset of effect, more predictable pharmacokinetic and pharmacodynamic profiles and avoiding the first-pass effect. The subcutaneous route of administration is highly preferable for many injectable drugs, such as trastuzumab, rituximab, bortezomib, granulocyte-colony stimulating factor, immunoglobulins, epoetin alfa and heparin. For some drugs (e.g. insulin), the choice of administration route depends on the clinical circumstances.1
Over the last decade, we have witnessed a dynamic increase in therapies that make use of biological drugs – medicines derived from living cells or through biological processes.2
Polypharmacy and dosing frequency appear to be rate-limiting factors in patient satisfaction and subsequent adherence.3 For self-injected drugs, the user interface plays an important role in the catalogue of factors that influence patient compliance, while reducing the complexity of the drug administration procedure has been highlighted as particularly important.4
“Nemera’s long-standing experience in the high-scale production of tailor-made pen injectors serves as a strong foundation and legacy that steers the company to go the extra mile, continuing its story.”
A PLATFORM APPROACH TO MEET KEY USERS’ NEEDS
In order to increase patients’ compliance and adherence, as well as to accommodate pharmaceuticals’ needs, developing a device platform needs to be strategically defined to ensure that it is designed for the maximum possible range of potential therapeutic areas and patient populations. This is to ultimately assure the device effectiveness. With this in mind, through user-evaluation studies, a platform should be tested by a broad, representative range of participants that reflects the diversity of potential device end-users. After performing this upstream work and early-stage diligence, a platform can be further examined using risk analysis to mitigate negative surprises in the late-stage development process.
Nevertheless, a platform should go through several adjustments and a few customisations to further translate it into a suitable combination product for both its target drug and the intended user population. The goal is to leverage baseline knowledge and generated data throughout the development of a device platform, then tailor it into a specific combination-product to meet pharma’s needs.
NEMERA PEN INJECTOR PLATFORMS: DESIGNED FOR VARIOUS THERAPIES AND APPLICATIONS
Innovative technological solutions that offer a simple and intuitive user interface are easily adopted by patients. This favours adherence to therapy as well as safe and reliable dose delivery. Furthermore, successful technology can be applied to the development of other medical devices.
In this way, the combination of technological solutions that ensure functional parameters are specified in defined ranges that provide a platform for the development of new products.
The use of the existing platform for the development of other pen injectors based on technological solutions invented in the course of the development of this platform is associated with a number of significant benefits, amongst which the following deserve special attention:
- Reduced development time
- Lower development costs
- Reduced risks of developing a new pen
- Implementation of a user interface with market-proven acceptability.
By fostering the platform approach, coupled with Nemera’s integrated end-to-end service programme offerings, the company helps its customers accelerate their specific combination product development programmes in the sprint to market.
Nemera’s long-standing experience in the high-scale production of tailor-made pen injectors serves as a strong foundation and legacy that steers the company to go the extra mile, continuing its story. Thanks in large part to the two strategic acquisitions made in the last couple of years, Nemera is well on its way to fulfilling its vision of becoming one of the most patient-centric drug delivery device companies worldwide.
Due to the acquisition of Insight Product Development in 2019, Nemera’s strength in early design research framework and human-factors strategy enables the company to leverage and tailor its product lines and platforms across different administration routes, including the recently acquired former Copernicus’s (Szczecin, Poland) pen platforms. Insight Innovation Centre (Chicago, IL, US) brings its experience from the earliest development phase. For example, within the US market, Insight helps navigate through the regulatory landscape to develop fitting solutions based on US FDA prerequisites (Figure 1).
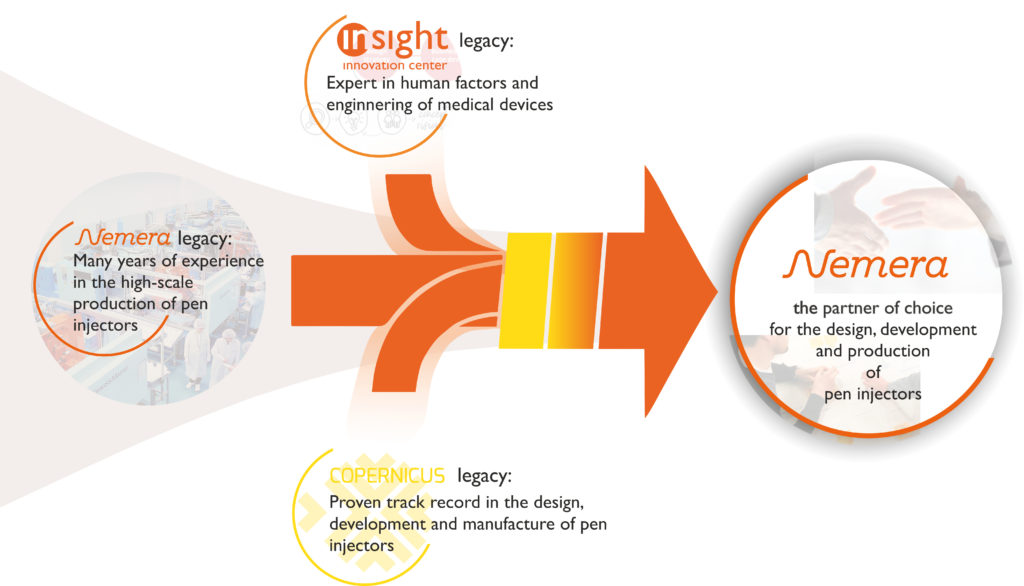
Figure 1: Comprehensive capabilities in the design and manufacture of pen injectors built through years of experience and targeted acquisitions.

Figure 2: Four pen injector platforms for various therapies to meet key user needs.
Furthermore, Copernicus’s proven track record in the design, development and manufacture of pen injectors boosts Nemera’s parenteral product portfolio and small series capabilities. The acquisition strengthens the company’s proprietary product platform offering and establishes an operations footprint in Eastern Europe. Nemera’s story continues to reinforce its focus and purpose of always putting the patient at the centre of everything it does by becoming the partner of choice for the design, development and production of devices, including pen injectors.
Acquired by Nemera in October 2020, Copernicus has developed four product platforms for pen development and manufacturing. These include PENDURA AD®, PENONE®, PENVARIO® and PENHV®, which are designed for reusable and disposable devices for various therapies to meet key users’ needs (Figure 2).
PENDURA AD® is Nemera’s product platform, dedicated to the manufacturing of market-proven, reusable pen injectors, which integrates an automatic, spring-driven feature coupled with a side-activation button. The range of products developed from the PENDURA AD® platform includes pen injectors dedicated to the administration of insulin and its analogues, growth hormones and PTH analogues, amongst others.
In the disposable segment, PENONE® has been marketed for its multiple-use fixed-dosing feature, including a dose counter in addition to the ergonomic side-activation button, as well as the automatic, spring-driven delivery. Thanks to the presence of a dose counter, the user of each pen from the PENONE® platform has ongoing control of the number of doses remaining in the pen. As a result, the PENONE® platform is regularly chosen for the development of pen injectors dedicated to various drugs with a fixed therapeutic dose.
Continuing Nemera’s story within the disposable pen injector space, its standard platform, PENVARIO®, is designed to be suitable for any drug with standard manual variable dosing. It is highly customisable for a wide range of applications, to match any drug administration. Thanks to its high flexibility in scaling production, Nemera is capable of meeting its customers’ needs in both low- and high-scale production. Along with the rise of high viscous drugs and larger-dose volume administration, Nemera identified the need for low-force spring-assisted disposable pen injectors designed for ease-of-use. Therefore, using its long-standing experience in spring-assisted pen injectors, Nemera has developed the PENHV® platform, which incorporates a spring-assisted, non-extendable push-button on top of the pen to facilitate seamless, low-force injection. It provides constant speed delivery for both fixed- and variable-dose pens and is highly customisable. Depending on the functional parameters defined together with the customer, a platform is selected that will be used to develop the pen injector with parameters strictly defined in the user requirement specification.
“Developing a pen device based on existing platforms facilitates the application of such technological solutions that can significantly foster patient adherence to the therapy, hence the competitive positioning of the drug used for administration with a given pen.”
Each product platform can be used to design pen injectors dedicated to both generic and innovative drugs. Having Nemera’s own design and development, own tool shop for the production of moulds, fully equipped plastic injection moulding setup and the possibility of both semi-automatic and fully automated production, means the company is able to provide a short time-to-market and cost-effective development of the pen injectors, based on its proven product platform, and to scale the production according to the actual customer needs.
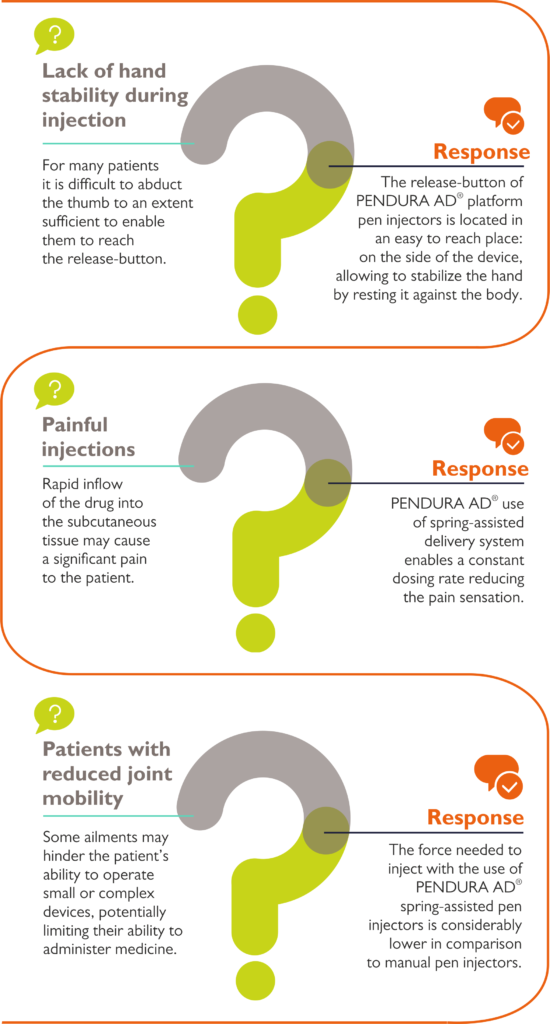
Figure 3: Improving patients’ adherence through ergonomic solutions.
CASE STUDY: PATIENTS’ BENEFITS TRANSLATED INTO SALES INCREASE
Innovative, user-friendly technological solutions invented during the development of the product platform are used in all pen injectors from a given platform. For this reason, the customer can be more confident both in terms of development timelines and its final results. Moreover, developing a pen device based on existing platforms facilitates the application of such technological solutions that can significantly foster patient adherence to the therapy, hence the competitive positioning of the drug used for administration with a given pen (Figure 3).
An example is the introduction of a pen from the PENDURA AD® platform to the European market. The manufacturer of the drug, whose domestic sales growth was three times lower than that of the market leader, decided to change the pen injector previously offered for use with its drug to a pen from the PENDURA AD® platform.
Within two years after the introduction of the pen injector from the PENDURA AD® platform, there was a decrease in the official drug price, which have could have significantly jeopardised the sales results of all the drug manufacturers. However, thanks to the introduction of the PENDURA AD® platform pen injectors, the sales value of the drug used with this pen increased, while the sales value of the company’s competitors significantly dropped. This example shows the value for the user of the pen injectors from the PENDURA AD® platform, as well as the role of the injection devices in the choice of the brand within a therapy area (Figure 4).
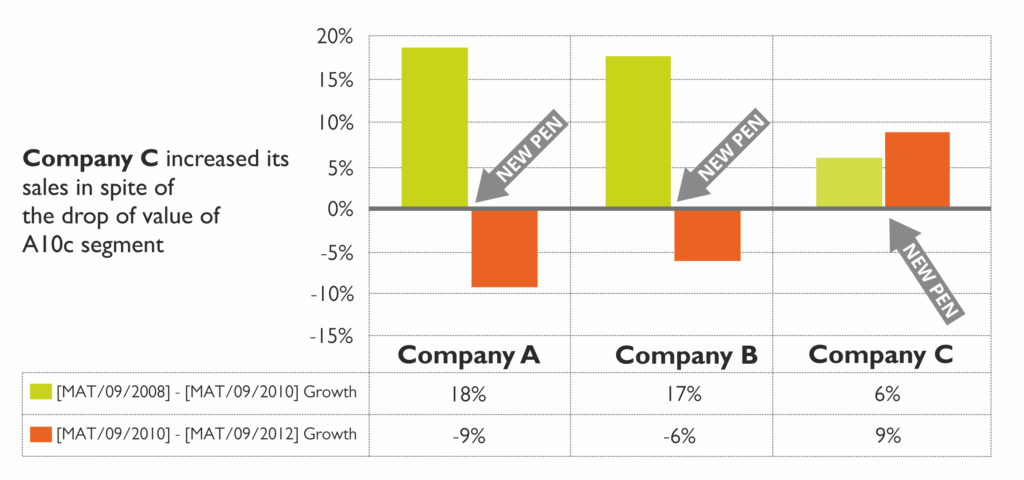
Figure 4: The impact of introducing a pen injector from the PANDORA AD® platform on the dynamics of drug sales.
It is worth mentioning that some detailed technological solutions can be imported to the new platform, dedicated to the production of other types of pen injectors. For example, the use of a technological solution that allows the ergonomic location of the dose-release button on the side of the pen, allowing the user to stabilise their hold on the pen by resting their hand against the body during dosing.
The positive assessment of this technological solution used in reusable pen injectors from PENDURA AD® platform, expressed by users in several post-market surveys, meant that the technological solution enabling the location of the dose-release button on the side of the pen was also used in the PENONE® product platform dedicated to disposable fixed-dose pen injectors.
NEMERA: YOUR PARTNER OF CHOICE IN DESIGN, DEVELOPMENT AND MANUFACTURING
With the integration of Copernicus and Insight Product Development, Nemera offers the highest quality standard for its customers and patients. The pen injectors are developed based on proven technological solutions, and their development is carried out by experts in the field of human factors engineering, design and production.
Looking to the future, Nemera is actively working on innovative platforms, by leveraging existing and discovering novel techno-bricks. Tapping into the converging trend of digital health and connectivity, the company is currently developing advanced solutions with the ultimate purpose of improving patient outcomes through offering robust and easy to- use solutions.
REFERENCES
- Jin J et al, “The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection”. Patient Prefer Adherence, 2015, Vol 9, pp 923–942.
- Sengupta A, “Biological Drugs: Challenges to Access”. Third World Network, Penang, Malaysia, 2018, pp 5-15.
- Boccara, F et al, “Practical Considerations for the Use of Subcutaneous Treatment in the Management of Dyslipidaemia”. Adv Ther, 2017, Vol 34, pp. 1876–1896.
- Zahn JD, “Analysis: desirable attributes of insulin injection pens that drive patient preference and compliance”. J Diabetes Sci Technol, 2011, Vol 5(5), pp. 1210–1211.

