To Issue 182
Citation: Quigg T, Al-Amari M, Gresham J, “The Platform Paradox: Device Development Challenges Within Fast Evolving Drug Discovery Pipelines”, ONdrugDelivery, Issue 182 (Jan 2026), pp 42–46.
Tim Quigg, Mariam Al-Amari and Joel Gresham discuss how to enable high-dose delivery for novel biologics, using ex vivo and in silico approaches to accelerate drug-device development and improve device performance, thus de-risking projects and saving costs.
There are a number of unmistakable factors that have propelled the success of platform devices. The ubiquitous push for at-home drug delivery has provided a clear and compelling business case for recalibrating drug discovery pipelines. Where biologic drugs were once almost exclusively delivered intravenously (IV), it is hard to ignore the increasing prevalence of subcutaneous (SC) assets across pharmaceutical pipelines. SC dosing regimens are now not only being developed in parallel to IV dosing, but increasingly being prioritised as the primary or standalone dosing option.
As many pharmaceutical companies normalise expansive pipelines with 100+ assets progressing through clinical milestones, the appeal of platform devices is clear; amortised device investments, with multiple assets benefitting from the same delivery device technology. While this “one-size-fits-all” elevator pitch is compelling, it may be in direct contrast to trends within large-dose biologics development.
Large-dose administration has seen the industry continue to challenge on-market precedents, incrementally pushing the perceived limits of acceptable drug volumes, viscosities and delivery times. Furthermore, many large-dose assets exhibit highly complex rheological behaviours (Figure 1). At one extreme, ultra-high concentrations are breaching the 200 mg/mL precedents that have been established over the last decade, even reaching beyond 500 mg/mL. These trends have coincided with the development of non-aqueous suspensions, where a small modification in formulation chemistry can result in significant changes to syringeability, drug delivery and even pharmacokinetic (PK) performance.
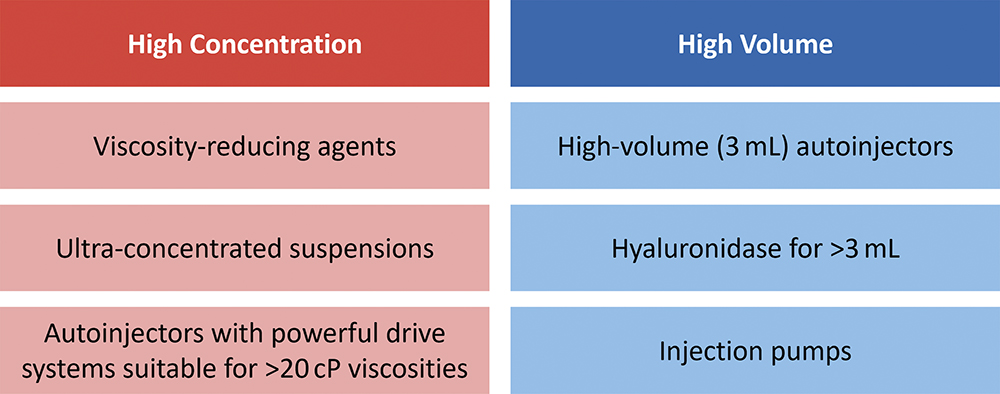
Figure 1: Opportunities for high-dose delivery are commonly segmented into opposing formulation strategies – high concentration and high volume.1
“DE-RISKING DEVICE DEVELOPMENT AND SELECTION WHILST WORKING WITHIN THIS EVER-CHANGING FORMULATION DESIGN SPACE HAS BECOME A KEY CHALLENGE FOR COMBINATION PRODUCT TEAMS.”
Conversely, at the other edge of the design space, the industry is seeing comparably large doses being achieved with higher volumes (Figure 2). Whilst 2 mL autoinjector administrations have become the norm in recent years, pipelines are pushing far beyond this theoretical limit, with the advent of large-volume autoinjectors and on-body injectors proving to be a catalyst for this change. De-risking device development and selection whilst working within this ever-changing formulation design space has become a key challenge for combination product teams.
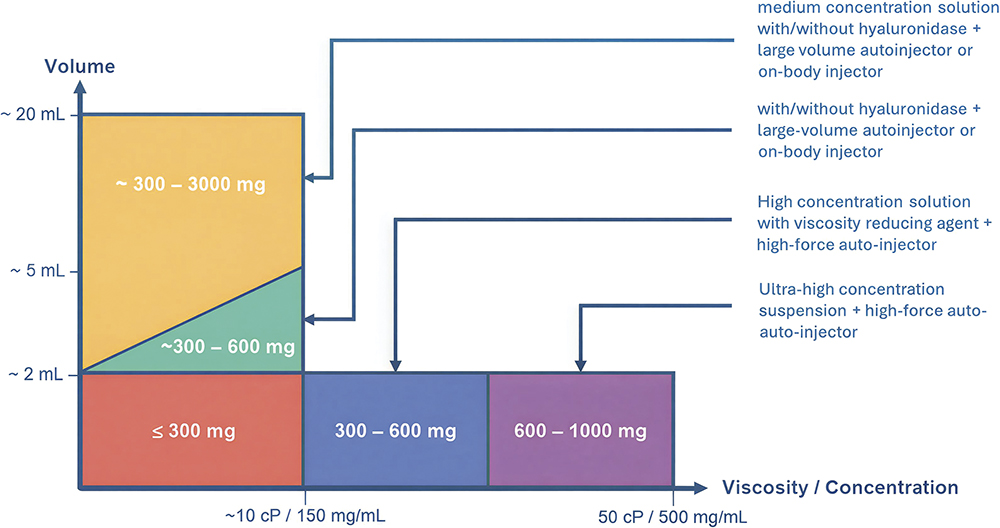
Figure 2: The high-dose design space is diverse, encompassing both higher volume and high concentration formulations, challenging a “one-device-fits-all” platform strategy.1
This can be described as “the platform paradox”. As off-the-shelf devices converge towards standardisation, next-generation biologics are pushing boundaries and allowing for increasingly unique, diverse design spaces. Thus, navigating this platform paradox requires novel research and development approaches, leveraging state-of-the-art analytical tools to make informed device decisions.
ENABLING HIGH-VOLUME DELIVERY
A variable that often remains unknown during device development is the behaviour of the injectate following injection. With the device landscape historically dominated by 1–2 mL or 1 cP prefilled syringes and spring-driven autoinjectors, it is no surprise that delivery profiles have often been overlooked. A 1 mL injection yields negligible skin distention and therefore characterising bolus formation is rarely deemed essential for traditional SC delivery. However, when going beyond the traditional design space – expanding delivery volumes up to 20 mL and considering high-viscosity formulation technologies – the dynamic behaviours of SC bolus formation become significant.
Most biologics are absorbed into the vascular or lymphatic capillaries prior to ending up in systemic circulation. As there are multiple biological barriers to cross before reaching systemic circulation, and due to their size, some biologics show unfavourable bioavailability profiles after SC injection. For instance, some studies have shown that needle depth upon administration affects absolute bioavailability and PK parameters for biologics.2 Therefore, this must be considered when developing injection devices for the SC route (Figure 3).
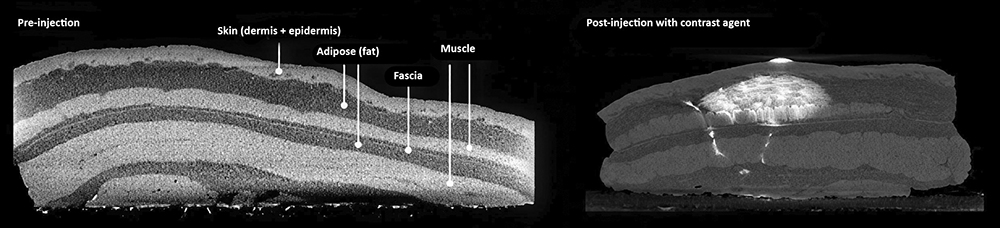
Figure 3: The SC region consists of adipose fat tissue, sometimes separated from muscle by the presence of fascia.
“CURRENT DEVICE PROGRAMMES CAN BENEFIT FROM SUPPLEMENTARY EX VIVO TISSUE TESTING, FRONT-LOADING THE CURRENT UNDERSTANDING OF DRUG-DEVICE-TISSUE INTERACTIONS PRIOR TO PRECLINICAL AND CLINICAL STUDIES.”
As bolus formation and PK performance become salient questions, traditional evaluation methods can prove both limited and costly. Preclinical and PK studies may be inevitable as a pipeline asset progresses towards market, but significant value can be derived through the adoption of ex vivo testing and advanced imaging. Where historic development programmes relied heavily on injections into air to demonstrate credible delivery times, current device programmes can benefit from supplementary ex vivo tissue testing, front-loading the current understanding of drug-device-tissue interactions prior to preclinical and clinical studies.
Despite traditional verification testing standards not requiring ex vivo evaluations, it is crucial to visualise depot formation and drug dispersion post injection. This, alongside measurement of tissue back-pressure, can help to guide device design (e.g. injection rate, needle selection, injection depth, push-on-skin pressure) and de-risk projects progressing towards the clinic.
Public domain literature presents the results of ex vivo testing on porcine abdominal tissue, leveraging micro-CT scanning technology.3 The porcine model was selected for its translational relevance to human tissue, with resemblant skin thickness and skin tissue architecture. While ex vivo tissue does not account for full injectate absorption due to its lack of lymphatic drainage and wider physiological behaviours, it is recognised that minimal fluid removal occurs within the first 20 minutes post injection, enabling an accurate study of injectate dispersion in SC tissue immediately after injection.3
A 3D injected bolus volume can be derived following injection, providing datasets that can be interrogated to identify statistically significant variables (Figure 4). Data from these 3D images can be used to assess industry-wide areas of uncertainty, from predicting bioavailability changes as delivered volumes increase, to how bolus morphology may influence patient tolerability and pain.
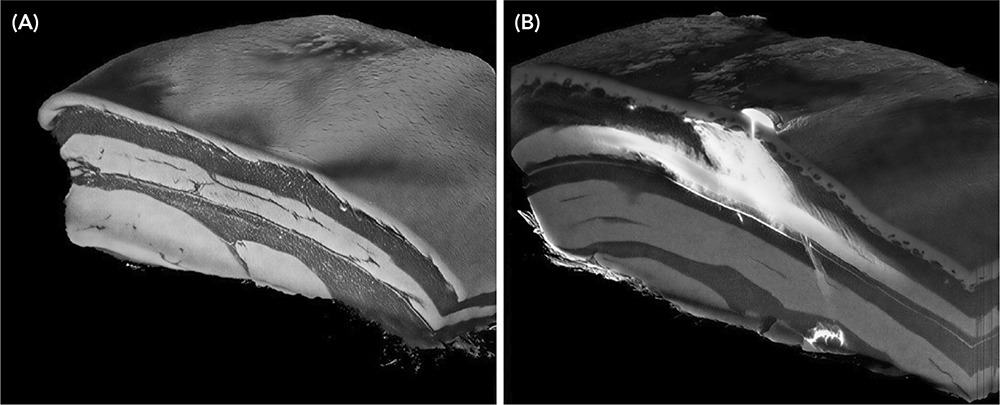
Figure 4: 5 mL bolus in ex vivo tissue, showing partial intramuscular penetration below the intended SC layer and significant leakage from the injection site. (A) Before injection. (B) After injection.
Early-stage studies began as qualitative, comparative evaluations of in-development formulations. Today, this approach has matured, with refined test protocols, tissue handling frameworks, imaging procedures and data processing for investigating bolus spread, injection site reactions, leakage and skin distention. With advancing research, it has been found that this data can feed directly into device development programmes to:
- Inform optimal injection sites for different patient populations, which has an immediate effect on drug PK
- Study pain and tolerability as a function of bleb formation and size to design injection devices that may improve patient adherence
- Evaluate how injection rate and viscosity can influence leakage after needle removal, resulting in injectate loss, which influences how long the injection duration should be
- Assess device-skin interactions and how large-volume administration may affect tissue backpressure.
Combining the data derived from micro-CT imaging to visualise the injectate dispersion post injection has proven to be a valuable method to enhance due-diligence processes and inform the design space for in-development combination products.
MODELLING THE HIGH-CONCENTRATION DESIGN SPACE
In silico modelling and simulation technologies can be used to inform and establish the design space for high-dose SC biotherapeutics. The high-concentration domain remains poorly defined due to the novelty of high-dose biotherapeutic formulations and a historical focus on small molecules. These typically do not exceed 2 mL dose sizes and remain below a concentration of 250 mg/mL. As formulation technologies mature, so too must analytical techniques.
Advanced simulation approaches using finite element analysis and computational fluid dynamics (CFD) can be deployed to create a digital twin to simulate the complex mechanics, material behaviours and fluid interactions of high-concentration therapies. Digital twins are already acknowledged for their ability to predict complex device functionality, though their utility may be extended to include drug-device interactions. This enables device engineers to collaborate closely with formulation teams, evaluating how in-development drug assets may perform with available platform devices. Further challenging the platform paradox, these digital approaches can be leveraged to define clear device performance limits across the emerging formulation design space.
Amongst today’s high-dose formulation technologies, suspensions remain prevalent. Whilst overall viscosities may be kept relatively low, suspension-based formulations are not without complexity when integrating them with platform devices. The most pertinent risk relates to poor syringeability (drawing into a syringe from a vial) and injectability (delivering out of a syringe into tissue) due to particle-induced needle occlusion. These issues stem largely from inter-particle interactions, including sedimentation and agglomeration. Whilst a fully mixed suspension avoids needle clogging, the risk is increased when a formulation is not fully reconstituted or resuspended before use (Figure 5).
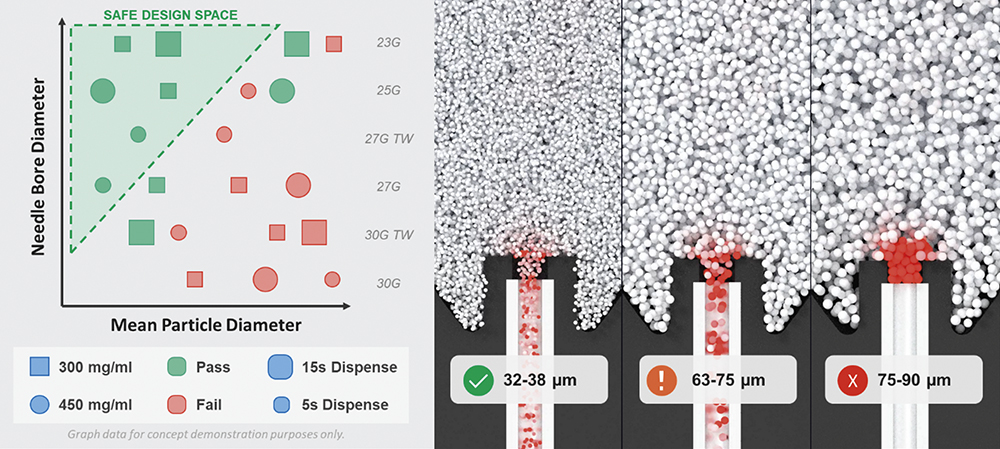
Figure 5: CFD and discrete element modelling to optimise drug particle and needle size specifications.
Addressing the significant complexity of biologics, with a diverse range of formulation variables, by conducting a comprehensive design-space investigation into needle occlusion would be costly and time-consuming. In contrast, a large virtual design-space study can be completed in a matter of weeks by leveraging computational models and high-performance computing. Hundreds of simulations can be run in parallel, exploring thousands of device-formulation combinations to inform development strategies. Such models can evaluate how factors such as particle morphology, cohesivity and vehicle rheology can directly influence needle occlusion. Combining these studies with the variability observed across the device landscape (e.g. needle size and inlet geometry) provides unparalleled insight into drug-device interactions when changing formulation properties.
REFERENCES
- Bruin G et al, “An industry perspective on clinical development and regulatory strategies for subcutaneously administered high-dose biologics”. J Control Release, 2025, Vol 386, art 114156.
- Hu P et al, “Systematic review of device parameters and design of studies bridging biologic-device combination products using prefilled syringes and autoinjectors”. AAPS J, 2020, Vol 22(2), art 52.
- Gresham J et al, “Visualisation and quantification of subcutaneous injections of different volumes, viscosities and injection rates: An ex-vivo micro-CT study”. J Pharm Sci, 2024, Vol 113(12), pp 3447–3456.

