To Issue 177
Citation: Barichello E, Ravazzolo V, “Two into One: Solving Complex Drug Delivery Problems with Dual-Chamber Containment Solutions”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 14–18.
Enrico Barichello and Valerio Ravazzolo describe how dual-chamber solutions ensure isolation, integrity and safety for managing lyophilised and liquid drugs. With innovative and customisable designs, these technologies improve drug delivery, reduce contamination risks and simplify complex therapies, addressing the evolving needs of both pharmaceutical industry and patients.
“DEVELOPED IN EITHER CARTRIDGE OR SYRINGE FORM, DUAL CHAMBER CONTAINERS PROVIDE A PLATFORM FOR ACCOMMODATING TWO INJECTABLE ELEMENTS THAT NEED TO BE KEPT SEPARATE UNTIL THE POINT OF USE.”
At the point of delivery, an injection is a fundamentally simple act – within a single process, the contents of a cartridge or syringe are expelled under force through a needle via the skin.
Advances in injectable technology have simplified this task further, in some cases even removing the requirement for administration to be carried out by a healthcare professional and handing responsibility to caregivers and patients themselves. By doing so, these methods enhance patient convenience and help to maximise the likelihood of sustained adherence to the dosing regimen.
However, not all injections are necessarily so straightforward. In some contexts, this process is complicated by a range of fixed parameters. For example, a drug might be lyophilised in the interests of shelf life and stability, but must undergo a process of reconstitution into suspension or solution before it can be injected. Alternatively, in the case of combination therapies, multiple drugs might need to be delivered concurrently but are not co-formulated into a single integrated product. In these instances, the desire for accuracy in dosing, simplicity in delivery and even the possibility of self-administration remain constant, but important challenges must be overcome for these ideals to be realised.
A SINGLE CONTAINMENT SOLUTION FOR SEPARATE INJECTABLE ELEMENTS
A frequently effective solution to this conundrum is the use of dual-chamber containment systems. Developed in either cartridge or syringe form, dual-chamber containers provide a platform for accommodating two injectable elements that need to be kept separate until the point of use, whether in the form of liquid–liquid drug product combinations or lyophilised powders that need to co-exist with a diluent for reconstitution immediately prior to delivery. In either case, containing the two entities together enables the patient to undergo a coherent and measured injection process, making a potentially complex scenario far more simple, streamlined and convenient (Figure 1).
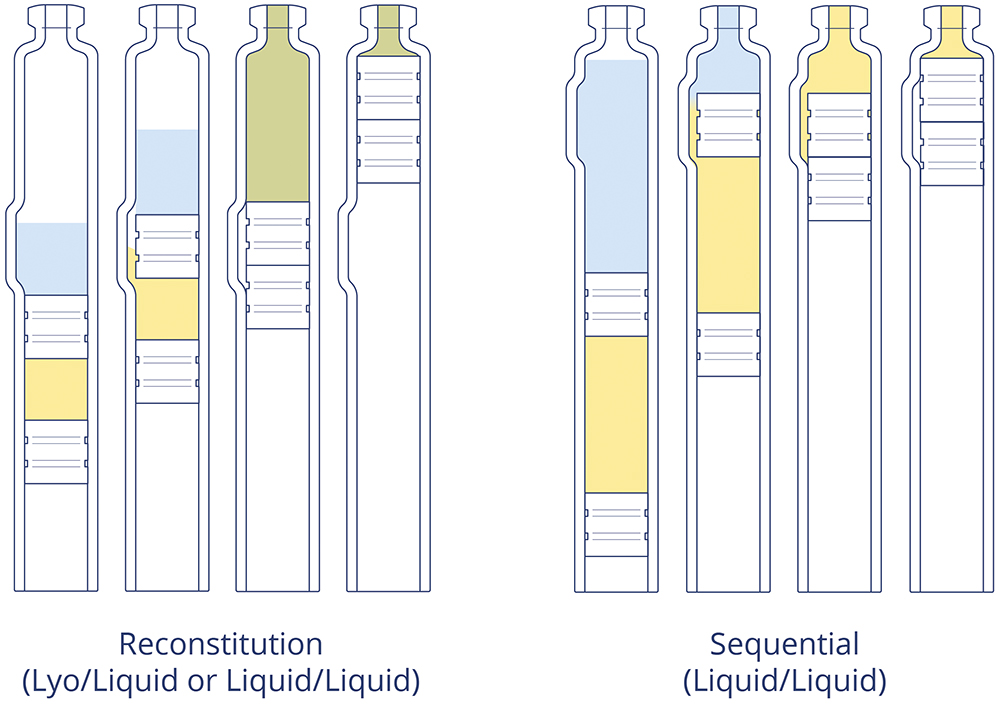
Figure 1: Dual-chamber containers can enable two delivery configurations: premixing and reconstitution of the drug product or sequential injection of two drug liquid products.
Demand for dual-chamber containment solutions has grown in line with advances in treatments for a wide range of indications, including growth hormone therapy, diabetes and schizophrenia.1 The dominant growth area, however, is weight management, where the dynamic rise in glucagon-like peptide-1 (GLP-1) receptor agonists continues unabated and co-administered drugs offer the potential to further increase their efficacy.
Combination therapies of this type must accommodate a range of factors, from the chemical stability of different drugs to the timing of delivery and patient usability. In some cases, the obligation to satisfy these limiting parameters can lead to an undesirable situation where patients must receive two separate injections of products that serve a complementary purpose. Dual-chamber containment systems offer a way to reconcile these factors by enabling the two distinct components to remain physically separate within a single delivery container until the point of administration.
A PLATFORM FOR BYPASSING CO-FORMULATION AND SIMPLIFYING RECONSTITUTION
For combination therapies where two agents must be delivered simultaneously but cannot be co-formulated due to chemical incompatibility or regulatory constraints, dual-chamber formats enable drug integrity and efficacy to be maintained without the need for a patient to undergo multiple injections. At the same time, they effectively circumvent the co-formulation process, which, if it is even possible, can extend timeframes and result in higher costs.
“EMPLOYING A DUAL-CHAMBER INJECTION SYSTEM SIMPLIFIES THE INJECTION BY INTEGRATING THE RECONSTITUTION STEP INTO A MORE COHERENT, MANAGEABLE AND UNIFIED PROCESS.”
Alternatively, where drugs are formulated in a lyophilised format, the requirement for reconstitution requires that multiple additional steps are completed before a patient can be injected or self-inject. Lyophilisation can be crucial to ensure that stability and potency are protected throughout the shelf life of a drug, which might otherwise degrade in aqueous suspension – an issue particularly associated with delicate biologics. Reconstitution, however, introduces additional time, complication and risk to the injection process, which, in turn, can compromise patient acceptance, usability and adherence. Employing a dual-chamber injection system simplifies the injection by integrating the reconstitution step into a more coherent, manageable and unified process, making injections not only faster but also reducing the likelihood of administration errors on the part of patients or caregivers.
There are two main containment configurations that are typically used – dual-chamber syringes and dual-chamber cartridges. Each offers specific functional benefits depending on formulation type, delivery route and device integration. Syringes are typically employed for liquid–liquid self-administration scenarios, as might be the case for autoinjectors. Cartridges, meanwhile, are suited to lyophilised–liquid scenarios, as well as liquid–liquid applications, and are ideal for pen systems and drug delivery platforms where repeated injections are required.
DESIGNED AND ENGINEERED ACCORDING TO DELIVERY REQUIREMENTS
In dual-chamber containment solutions, the design of the barrel structure is critical to the chosen injection process, and careful consideration must be given to both the position of the bypass that facilitates mixing and the position of the middle plunger that separates the two elements. When pre-mixing is required, the bypass is located in the middle of the cylinder barrel. Under pressure, the contents move along the container until the separating plunger aligns with the bypass “bulge”. At this point, the first element can enter the bypass and combine with the second element nearest the needle. After mixing is complete, the combined formulation can then be injected as a single dose.
In sequential delivery, where two components must be injected one after the other without prior combination, the bypass is positioned as close as possible to the shoulder of the barrel. Here, the injection force pushes all contents along the container, first dispensing the full dose of the primary liquid. When this phase is complete, the separating plunger will be aligned with the bypass, thus enabling the secondary liquid to be forced around its side for subsequent delivery as part of a single action.
Whether in the case of sequential injection or pre-mixing, both configurations require meticulous engineering from a glass-forming perspective. Specific parameters, such as the volume of the chamber contents and plunger dimensions, will have an impact on the position and length of the bypass required. Factors such as drug viscosity, fluid pressure and glass stress must also be taken into account, as resistance forces will be higher in injection systems employing two plungers. In addition, there can be bespoke customer requirements around plunger selection to satisfy barrier performance and provide sterility assurance, specifically for the separating plunger in contact with two different products. Taken together, the multiplicity of these variables will require a degree of tailoring for the containment solution so that it precisely meets the needs of the application in question.
For lyophilised products, the lyophilised cake must remain physically and chemically isolated during storage, avoiding contact with liquid to prevent activation. As such, the glass barrel and plungers must together maintain a sterile barrier that can endure long-term storage and transportation.
Critical to performance is the seal integrity of each chamber and the ability of the internal plunger to pass through the container without compromising sterility. The reconstitution step must also be both effective and relatively effortless for the end user, while also avoiding the risk of contamination and ensuring even dispersion.
MAINTAINING INTEGRITY FROM POINT OF MANUFACTURE TO POINT OF DELIVERY
With these considerations in mind, a detailed appreciation of the fill-finish process is required in dual-chamber containment applications. Specifically, there is a clear need to maintain separation between either a lyophilised powder and liquid diluent or two liquid formulations, which can be a technically demanding task. Filling and sealing must also be completed with careful consideration of the potential for both cross-contamination of chamber contents and contamination by the external environment. This is a key reason why cartridges suit lyophilised material, as the container can be filled from the neck, isolating the drug from the activation liquid before freeze-drying takes place. Syringes, however, require filling via the plunger end of the cylinder, potentially bringing powder into contact with upper parts of the cylinder, which should be exclusively reserved for the activator liquid.
Regulatory scrutiny also underlines the need to maintain the integrity of both chambers and, indeed, the system as a whole. Assurances are required of the compatibility between materials and the avoidance of contamination risks, including from extractables and leachables. Dual-chamber systems must also guarantee performance throughout their shelf life, withstanding environmental stress, mechanical handling and prolonged storage while maintaining biocompatibility. In the case of prefilled syringes containing lyophilised drug and diluent, guidelines from the US FDA specifically highlight the importance of ensuring system integrity and undertaking comprehensive drug-device compatibility assessments.2
A DIVERSE SERVICE OFFERING DEDICATED TO DUAL-CHAMBER SYSTEMS
The breadth of Stevanato Group’s service offering allows the company to address these requirements and manage such complexity via an integrated approach. While centred around the company’s proprietary glass-forming technology, its offering is supported by deep expertise in drug containment, including analytical services to de-risk container and device selection, providing confidence from development though launch. The benefits of this approach are exemplified in Stevanato Group’s development of a special container bypass design. This configuration complements the company’s more traditional dual-chamber container offering, where the addition of the bypass channel manifests as a bulge, laterally extending the container profile. However, with the possibility that this asymmetric profile could add burden to handling and processing tasks in fill-finish, or that it could present issues of compatibility in device integration, Stevanato Group has engineered an internal multi-bypass configuration. This option maintains the symmetric, circular container profile while facilitating transfer between the chambers through the inclusion of multiple smaller-scale bypass channels (Figure 2).

Figure 2: Stevanato Group’s internal multi-bypass configuration facilitates transfer between the chambers through the inclusion of multiple, smaller-scale bypass channels.
“DRAWING ON ITS EXPERTISE IN MODERN FILL-FINISH OPTIMISATION, STEVANATO GROUP IS ALSO ABLE TO SUPPLY DUAL-CHAMBER CONTAINERS READY-TO-USE IN ITS
PRE-STERILISED EZ-FILL® CONFIGURATION.”
These glass engineering capabilities are complemented by Stevanato Group’s in-house manufacturing capability, which can accommodate production of bespoke container designs, enabling the company to develop cartridges and syringes in a range of configurations tailored to both drug characteristics and device integration needs. This includes options for liquid-lyophilisation and liquid–liquid formats, with full support for sequential or pre-mix delivery modes. Drawing on its expertise in modern fill-finish optimisation, Stevanato Group is also able to supply dual-chamber containers ready-to-use in its pre-sterilised EZ-fill® configuration. Taken together, these measures all contribute to a streamlining of downstream development, helping accelerate time to market.
TWIN BENEFITS: LIFE-ENHANCING THERAPIES PLUS PATIENT-CENTRIC DELIVERY
Indeed, it is with market-readiness in mind that Stevanato Group’s dual-chamber containment offering has evolved to this point. The platform has been shaped not by purely scientific curiosity, but by direct collaboration with pharmaceutical partners navigating today’s therapeutic frontiers, where ease of delivery remains paramount for patients in self-administration applications. However, it may be that reconstitution of a drug is required or that co-formulation of two drugs is chemically or temporally unviable.
In these scenarios, dual-chamber containers offer an elegant, adaptable and pragmatic method for solving a potentially challenging equation. Consolidating multiple preparation steps into a single injection motion supports dosing accuracy, reduces errors and enhances adherence to therapies that might otherwise require clinical oversight. And, as care continues to shift increasingly towards personalised medicine and patient-centric delivery, dual-chamber solutions look set to play an ever more important role in enabling complex, innovative therapies to be matched by simple, safe and effective delivery at the point of need.
REFERENCES
- Ingle RG, Fang W-J, “Prefilled dual chamber devices (DCDs) – Promising high-quality and convenient drug delivery system”. Int J Pharm, 2021, Vol 597: 120314.
- Draft Guidance for Industry and FDA Staff: Glass Syringes for Delivering Drug and Biological Products: Technical Information to Supplement International Organization for Standardization (ISO) Standard 11040-4. US FDA, April 2013.

