To Issue 155
Citation: Draper P, “Unlocking the Potential of Connected Drug Delivery Devices for Diabetes”. ONdrugDelivery, Issue 155 (Dec 2023), pp 12–15.
Paul Draper discusses the current state of connected injection pens and devices in the diabetes space, exploring the benefits that connectivity can bring to drug delivery devices and considering how to exemplify good design in a connected injection device for diabetes drug therapies.
“The aim of connected devices in diabetes is simple – to empower patients and healthcare professionals with accurate data to improve their ability to achieve better glycaemic control, through increased adherence and persistence with insulin therapies.”
Diabetes is a chronic condition in which the body does not produce sufficient insulin, or use it properly, potentially leading to severe health implications. To help prevent these problems, many people rely on insulin therapy, which can require administration of multiple doses of different insulins every day. Frequent adjustments to insulin dosage are commonly needed to accommodate variations in diet or lifestyle. Keeping track of all these factors and adjustments can be challenging, particularly when trying to manage diabetes alongside the other complexities of daily life.
A chronic condition with a patient-adjusted treatment regimen will carry clear and significant challenges to patient adherence. Notwithstanding the challenge in accurately determining real-world adherence rates, studies have indicated that adherence rates for insulin therapy could be as low as 44.3% in Type 2 diabetes patients.1 Furthermore, studies have shown that only 20% of people starting a basal insulin treatment plan continued beyond the first year.2 What’s more, these figures are unlikely to fully capture those patients who miss doses or take incorrect dosage values. Whatever the true figures are, there is, undoubtedly, a clear problem with patients lacking the control they need to avoid the secondary health complications that inevitably arise from inadequately managed diabetes.
The majority of patients deliver insulin therapy with pen injector devices (59% in the US and 93.6% in Europe) rather than syringe and vial systems.3 Through continual optimisation, the design of these pens has matured over the last 20 years but, today, these classical mechanical pen injectors and advances in pharmaceutical molecules may well be reaching the top of their respective developmental ‘S-curves’, leaving only modest opportunity to improve outcomes without a change in the model. This is where connected insulin delivery devices can now make the difference.
The aim of connected devices in diabetes is simple – to empower patients and healthcare professionals with accurate data to improve their ability to achieve better glycaemic control, through increased adherence and persistence with insulin therapies. Indeed, implementing connectivity has long been a target of pharmaceutical and drug delivery device companies more broadly. However, to date, relatively few connected devices have been commercialised, and those that have been have often added significant usability burdens for patients, which limits their appeal and means that uptake is unlikely to be widespread.
For diabetes specifically, the advantages for patients, payers and insurance companies alike can be shown by examining the total cost associated with related treatments. Data from UK expenditure published in a 2011 study is illustrated in Figure 1, showing that, in total, £13 billion was spent on diabetes treatment. Of this, only £1 billion was on the cost of drugs for direct treatment.4 The majority of the rest of the costs are associated with the treatment of complications. As such, it can be argued that the opportunity already exists to reduce the burden on the healthcare and insurance systems using connected devices.
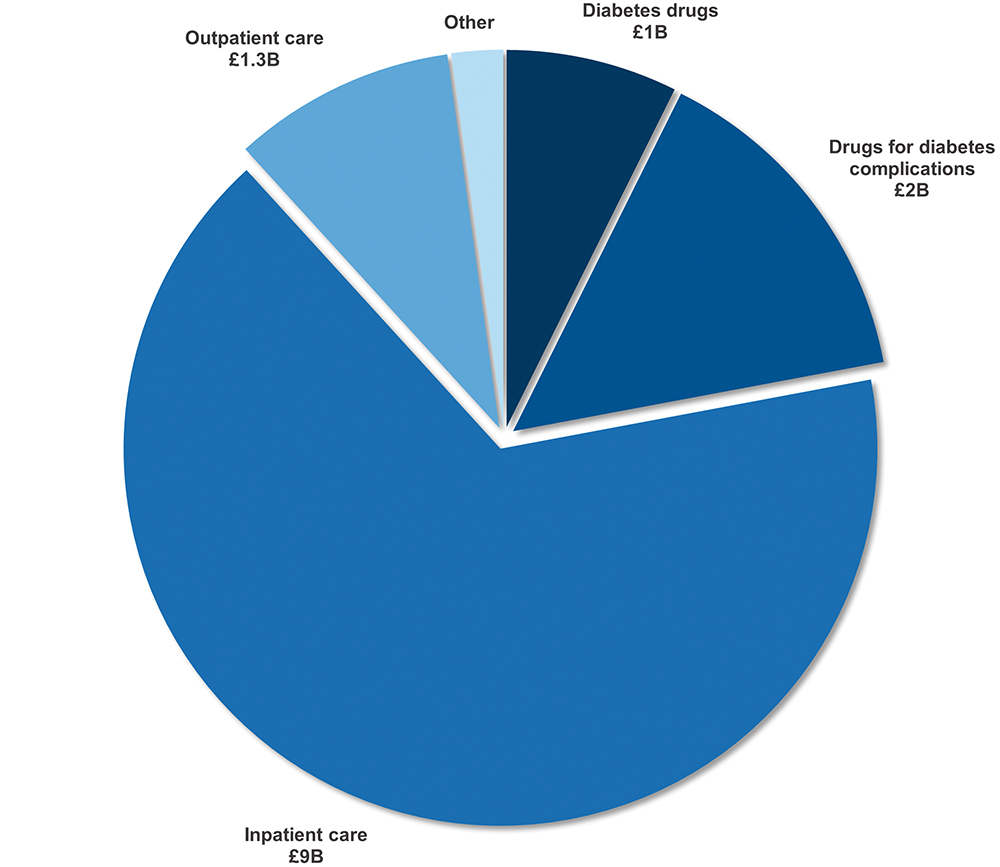
Figure 1: UK healthcare cost for treatment of diabetes and complications.4
The upside for pharmaceutical companies can be expressed relatively simply as well. If patient adherence and persistence is low, then less insulin is being purchased. This is magnified if one considers that prescribers may be more likely to switch to an alternative insulin product that has a compliance-aiding connected device if a patient is not achieving good control of their condition.
Therefore, it is arguable that the potential value of connected insulin delivery devices to patients, healthcare providers, payers and pharmaceutical companies is compelling. Hence, the challenge remains to develop devices that are capable of taking maximum advantage of this value by addressing the needs of patients within a framework that also considers the overall cost to healthcare systems.
“It is important to make usability the central factor when considering
the level of adoption, and ultimately the success, of an injection device.”
A CONNECTED ECOSYSTEM
The increasing rates of adoption of continuous glucose monitors (CGMs) could be considered to be paving the way for highly effective diabetes management with connected insulin injection devices.5 CGMs illustrate why usability is a critical factor for patient adoption. One of the key reasons why CGMs are popular is the reduction from one finger “stick” per reading to one “stick” every 14 days. Note also that the latest generation of CGMs seems to be tending towards fully seamless data synchronisation via Bluetooth, also demonstrating the importance of simplified user interaction.
For the most part, the benefit of connecting pen injectors themselves comes down to management of information. The right information, well-presented, can inform decision making and more timely interventions, ultimately leading to better outcomes (Figure 2). For some people, it could be said that the ideal for diabetes treatment would be a fully closed-loop system, with a body-worn insulin pump working in conjunction with a CGM to form a pseudo “artificial pancreas”.

Figure 2: The information-to-outcome pathway.
However, algorithm-controlled dosing may not be the perfect fit for everyone, and losing control of therapeutic decisions may be of particular concern for some physicians. A fully wirelessly controlled delivery device, such as a pump, also carries the downsides of a likely higher upfront product cost and cybersecurity vulnerabilities. As a balanced option for growing patient populations, connected injection pens are a compelling alternative – indeed, studies have been published with some of the latest connected injection devices demonstrating clinically measurable benefits.6
THE ADOPTION EQUATION
The level of interest, and ultimately adoption, can be said to follow a relatively simple equation (Figure 3). The primary consideration is the value to the individual patient, which will differ from person to person. However, it should also include metrics such as convenience and peace of mind, as well as the health outcomes that provide value to the patient as well as the healthcare providers and payers.
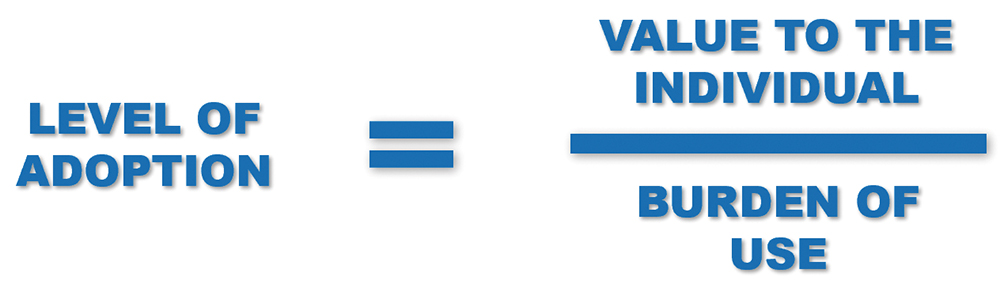
Figure 3: The adoption equation.
Similarly, the “burden” will also change from person to person and is likely to include aspects such as the full-life fiscal cost to the patient or payer, environmental impact, number of task steps required, the complexity of those tasks and the time overhead per patient for the healthcare professional, among others. For connected, or smart, devices to succeed, the equation must be balanced such that the value proposition outweighs the overall burden.
“The lowest power and easiest technologies to implement inherently carry additional user burdens.”
THE SEVEN PILLARS OF CONNECTED DRUG DELIVERY DEVELOPMENT
It can be helpful to categorise the aspects of device development into seven topics to illustrate the key things that need to be considered both to maximise the value and reduce the burden (Figure 4). This article will reference just a few of the key topics from the seven, in order to highlight some important considerations.
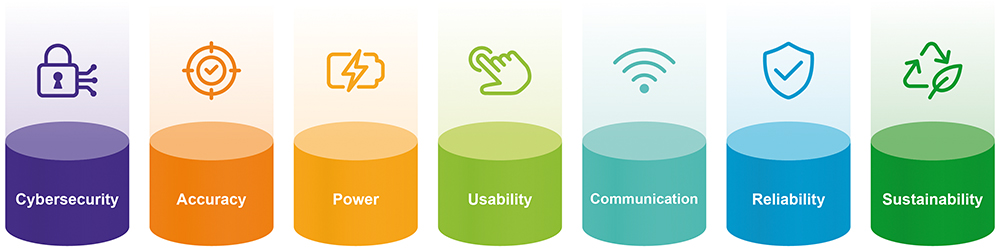
Figure 4: The seven pillars of connected drug delivery development.
It is important to make usability the central factor when considering the level of adoption, and ultimately the success, of an injection device. It is often all too easy to make compromises to usability when seeking to design connected injection devices or device accessories. A few examples of the problems associated with these compromises are discussed below.
Insulin pen injectors are often optimised during design to balance the thumb extension, for single-handed operation, with the dispense force (note that increased mechanical advantage brings lower dispense forces but longer travel distances). It is often simplest to integrate the connectivity hardware into the button of the insulin pen, conveniently encoding the mechanism for dispensing recording, for example. Figure 5 illustrates how this can be detrimental to the device’s ergonomics and can start to exclude some user groups from dispensing how they want to and have been able to historically.
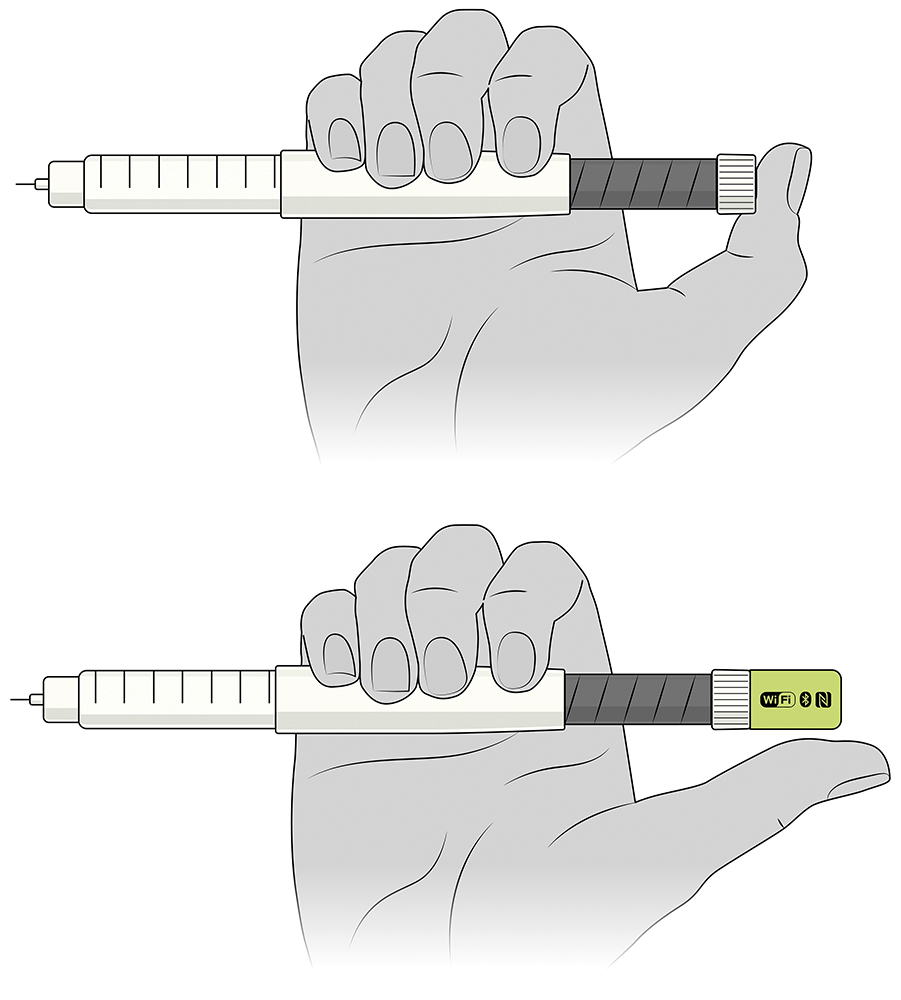
Figure 5: Illustration of the potential impact of integrated electronics.
Further to considering the impact on existing steps or user actions, there is the consideration of additional use steps. The clear best-case scenario here is a seamless user experience, meaning a solution that can be implemented in a way that adds virtually no additional user steps. This will likely require greater innovation and engineering expertise during development but may, ultimately, be a key factor in driving the success of the product.
When considering adding electronics to an injection device, it will, realistically, require the incorporation of a battery and, therefore, it is necessary to make choices concerning what battery technology to use, as well as battery charging and replaceability. There is significant user overhead associated with charging, in both the physical action of connecting cables or docks and the cognitive aspects of interpreting signals from the device and remembering to charge at the appropriate times.
The alternative approach of making the battery replaceable by the user leads to all sorts of usability challenges for accessing and handling small batteries and casework covers. However, with leading-edge engineering development and innovation in low-power solutions, it is possible to implement a battery that lasts the lifetime of the injection device, thereby requiring no specific intervention from the user above and beyond the normal use of the device.
Another key topic is data communication technology. It is a fundamental fact that, to make a connected solution, data connectivity is required. Again, seamlessness is incredibly powerful here. However, communication is heavily linked to power usage, as well as usability, and cannot be considered in isolation.
The lowest power and easiest technologies to implement inherently carry additional user burdens. A wired or optical data download will require the user to take steps to connect cables or docks and to initiate the data transfer from either side, or both sides, of the communication link. Similarly, the use of near-field communication (NFC) technology may require slightly fewer user steps, but still requires a deliberate and conscious action to be taken. On the other hand, a longer range wireless technology, such as Bluetooth, can be made to operate seamlessly in the background. However, this comes with a greater power requirement and therefore adds to the development challenge. That said, the ultimate reward for overcoming this challenge is likely to be measurable in the adoption rates for the product in market.
CONCLUSION
Designing connected drug delivery devices that can succeed is a complex and multifaceted challenge. It is a key strategic requirement to understand the business case for the device, as well as its value proposition to the patient. It is then even harder to design and develop a connected device that preserve these strategic goals without accepting compromises at some point in the process. Success requires a truly integrated approach, from strategy, human factors, mechanical engineering, electronic engineering, software engineering and industrial design, to manufacturing and industrialisation. All of these aspects need to be led by decision makers with the vision and experience to forge a path to success.
REFERENCES
- Perez-Nieves M et al, “Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US”. Curr Med Res Opin, 2016, Vol 32(4), pp 669–680.
- Yavuz DG, Ozcan S, Deyneli O, “Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens”. Patient Prefer Adherence, 2015, Vol 9, pp 1225–1231.
- Masierek M et al, “The Review of Insulin Pens-Past, Present, and Look to the Future”. Front Endocrinol (Lausanne), 2022, Article 827484.
- Kanavos P, van den Aardweg S, Schurer W, “Diabetes expenditure, burden of disease and management in 5 EU countries”. LSE Health, Jan 2012.
- “2021 Annual Report”. Abbott, Mar 2022.
- Adolfsson P et el, “Increased Time in Range and Fewer Missed Bolus Injections After Introduction of a Smart Connected Insulin Pen”. Diabetes Technol Ther, 2020, Vol 22(10)m pp 709–718.

