To Issue 137
Citation: Lange J, “Wearability and Usability of the YpsoDose 10 mL Patch Injector”. ONdrugDelivery, Issue 137 (Sep 2022), pp 23–26.
Jakob Lange discusses the results of a recently published study into the usability and wearability of the YpsoDose 10 mL patch injector, providing a summary of the insights acquired during the study.
“Ypsomed conducted a study to examine the feasibility and user perceptions of patch injector self-injections using an YpsoDose prototype.”
INTRODUCTION
The YpsoDose is a large-volume patch injector that delivers a single-dose volume of up to 10 mL from a cartridge-based primary container, developed in response to the significant increase in demand for new large-volume subcutaneous therapies. These new therapies are usually antibody based and have payloads of up to 1000 mg, requiring a 5–10 mL fill volume. These therapies include treatments for autoimmune diseases, and orphan and rare diseases, as well as immuno-oncology drugs.
In various comparative studies, it has been confirmed that patients and healthcare providers (HCPs) prefer a prefilled and preassembled device, in large part due to the increased convenience. Beyond the immediate advantages of convenience, safety, assurance of the correct dose and time saved, pharma companies particularly like that the user is unable to manipulate the drug container before or after the injection event. Ypsomed has drawn on its experience with prefilled pens and autoinjectors to develop a modular and customisable platform to speed up time-to-clinic, lower up-front investment and lower project risk for pharma partners.
As there is little information publicly available on the usability and patient perceptions of large-volume patch injector technologies such as the YpsoDose, Ypsomed conducted a study to examine the feasibility and user perceptions of patch injector self-injections using an YpsoDose prototype. Specifically, data on usability (i.e. overall handling) and wearability (i.e. acceptability of size and weight) were collected from simulated self-injections. During a non-interventional observational study, 23 participants, including patients and HCPs, simulated the use of a large-volume patch injector, performed predefined movement and activity tasks, and reported their experience in terms of device usage, comfort while moving and confidence while wearing the device. The disease states of the patient participants are presented in Table 1. The complete study has been published in “Medical Devices: Evidence and Research”1 and is presented here in summarised form.
| Disease | Cancer | Multiple sclerosis | Rheumatoid arthritis | Psoriasis | Crohn’s disease | Asthma/COPD | Cardiovascular disease |
| Number of patients | 5 | 2 | 7 | 2 | 1 | 3 | 1 |
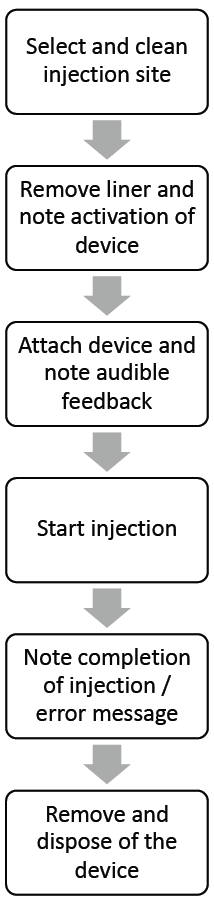
Figure 2: User steps required
to perform a simulated
injection with the YpsoDose
patch injection device.
*Certain patients suffered from more than one chronic condition
Table 1: Chronic disease states of patients (n=17)*.
METHOD
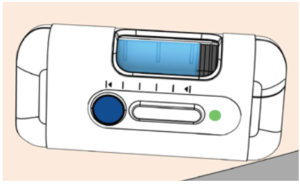
Figure 1: Prototype YpsoDose injection device used in the simulated use study.
Two injections were simulated for each participant using an YpsoDose patch injector prototype device as illustrated in Figure 1. The device is placed on the abdomen or thigh and held in place with a medical-grade adhesive. A single button activates the device after placement on the skin, with a sensor that determines when it is in contact with the skin. The device automatically inserts the needle when activated. It contains an LED-based progress bar and status lights, as well as a red warning light and audible indicator to indicate an error state. The injection progress is visible through a clear window on the device that shows the drug container and movement of the plunger.
The devices used in the study were fully functional, including the aforementioned lights, sounds and sensors. However, the devices did not contain a needle or liquid filling. The user steps required to simulate an injection with the study devices are shown in Figure 2.
RESULTS
General Impression
Participants responded favourably to the patch injector prototype, citing an expected advantage in terms of convenience compared with their current standard of care were they to use it to take their medication regularly. Multiple participants recognised the value of using a large-volume patch injector to enable them to administer their therapies at home, which would otherwise require them to be in a clinical or hospital setting. This observation was particularly pronounced among oncology patients, who welcomed an alternative to their current complex treatment process and the considerable time commitment it requires in relation to visiting inpatient care and infusion centres.
Usability
Regardless of their body size and shape, participants were able to find sufficient space to place the device and generally found wearing it to be comfortable and secure. In general, only a few user errors and difficulties were observed. During the simulated injections, participants were able to clearly identify that the injection process was proceeding correctly and verify the progress and status of the delivery. All participants were able to determine the state of the patch injector by checking the visual progress indicators (i.e. the visual feedback lights and plunger movement in the drug observation window). Most participants did not have any issue detecting the occurrence of an error state. Participants were able to remove the device without difficulty and knew that it had to be discarded in the sharps bin. The overall success rate of participants completing the simulated injections is illustrated in Figure 3.
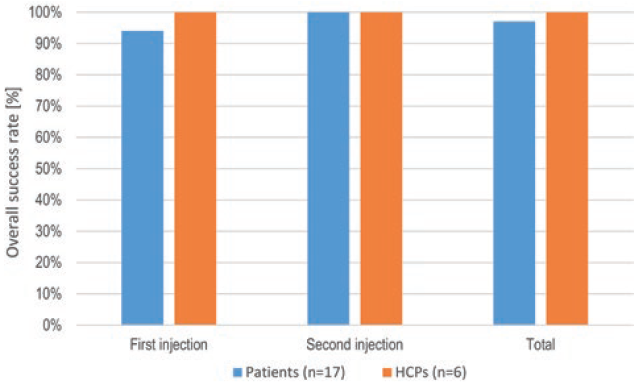
Figure 3: Injection success rates per injection and overall. An injection was rated to be successful if the user was able to successfully complete a complete simulated injection, regardless of usage errors or difficulties.
“The primary findings were that, although participants generally had a positive first impression of the device and injection technology, they were initially rather sceptical about the size of the device.”
Wearability
In general, patient participants were positively surprised by how easily the patch injector could be worn on the body during the simulated injections. The only issues related to wearability that were noted in the study were in cases of excessive body hair and small body size. For patient participants, the comfort and security of the patch injector were tested through multiple movement-based tasks during the simulated injection, with almost universal success across participants. Moreover, participants indicated that the patch injector was easily forgotten whilst performing the tasks.
Wearers quickly reached the point of sensory satiety, notably more so when the device was attached to the abdomen, rather than the thigh. This is a positive attribute, as the device does not seem to call attention to itself, either because of its size or a potential pull from the adhesive whilst moving. Thus, despite perceiving the device to be large, participants reported that the patch injector was comfortable when worn.
The ratings for abdomen and thigh placement in terms of comfort were almost identical, whereas removal was rated to be somewhat easier when the device was worn on the thigh. The participant ratings on perceived security while wearing the device were marginally lower than the comfort ratings, with participants viewing the abdomen as a more secure spot for the patch injector than the thigh.
SUMMARY & CONCLUSION
This study investigated the usability and wearability of a wearable patch injector, simulated with a prototype YpsoDose device. The primary findings were that, although participants generally had a positive first impression of the device and injection technology, they were initially rather sceptical about the size of the device. In terms of usability, participants were predominantly successful in performing the simulated injections with few user errors or difficulties. The self-reported rating for usability was high. In terms of the wearability, all participants were able to place the device on their bodies, irrespective of body size or habitus, and found the injector comfortable to wear. Participants were able to move around and perform predefined activity tasks with the device attached to them and rated the different wearability aspects positively.
In terms of self-reported usability, HCPs provided lower ratings than patients, although all ratings were high and the differences between the groups were small. Understanding the perspectives of HCPs is particularly important, as they are likely to continue to be involved in their patients’ care even with a move from clinic-based intravenous infusions to subcutaneous drug administration using novel large-volume patch devices. This is particularly true in oncology, where the development of subcutaneous formulations has recently attracted significant interest.
The choice of the injection site (abdomen or thigh) had a limited effect on perceived wearability. Patients rated most aspects of perceived wearability the same regardless of the injection site, with the exception of perceived security of attachment, which was rated higher for the abdomen, and ease of removal, which was rated higher for the thigh. These differences may be related to the size of the device, which is designed to hold a 10 mL cartridge.
These results indicate that the YpsoDose large-volume patch injector can be safely and effectively used by the intended broad patient and HCP population.
REFERENCE
- Lange J et al, “Formative Study on the Wearability and Usability of a Large-Volume Patch Injector”. Med Devices (Auckl), 2021, Vol 2021(14), pp 363–377.


