To Issue 133
Citation: Prestrelski S, “XeriJect™: Non-Aqueous Formulations for Subcutaneous Injection of Ultra-High Concentration Biologics”. ONdrugDelivery, Issue 133 (May 2022), pp 26–29.
Steven Prestrelski introduces the XeriJect non-aqueous formulation technology that creates formulations with visco-elastic properties, opening the door to subcutaneous injection of difficult-to-formulate biologics, such as monoclonal antibodies, at very high concentrations.
“Since their introduction, mAb therapeutics have had tremendous impact, but have yet to reach their full potential, largely because of hurdles in product formulation and delivery.”
Biopharmaceuticals are increasingly making up a larger fraction of the overall pharma market. In fact, over the past several years, sales of monoclonal antibodies (mAbs) have grown faster than all other biopharmaceutical classes. Global sales of mAb-based products are expected to grow to US$240 billion (£192 billion) by 2023 and $315 billion by 2025.1 Further, in recent years, multiple mAbs with similar drug targets, and biosimilars, have also entered the market. This creates a highly competitive product landscape compelling biopharmaceutical companies to differentiate their products. Since their introduction, mAb therapeutics have had tremendous impact, but have yet to reach their full potential, largely because of hurdles in product formulation and delivery.
To achieve therapeutic efficacy, antibodies can require doses as high as 1 g. Thus, mAbs have conventionally been administered via high-volume intravenous (IV) infusions that can last several hours and require administration under clinical conditions.2 Such infusions are inconvenient, and often financially inaccessible for the patient. Additionally, they can limit the number of patients that hospitals and infusion centres can treat. A preference for SC delivery is reflected in the market as an increasing number of mAb therapeutics have been released in as SC injections.3
Ready-to-use subcutaneous (SC) injections of biologics are preferable to IV infusions as they decrease the burden on healthcare providers and payers by requiring much less time and offering a lower risk of complications, such as infection and infusion reaction. For patients and caregivers, they offer the opportunity for self-administration and favourably improve the economics of drug administration. SC-administered mAbs can also be more affordable as they eliminate the high costs typical of in-clinic or at-home IV infusions.3
“These visco-elastic suspension formulations achieve maximally high drug loadings and are ready to use due to lack of particle settling on storage.”
Many mAbs, however, require high concentrations (>100 mg/mL) to administer efficacious doses within the volume limit of SC injection (1.0–2.25 mL). High concentrations in an aqueous environment are often intractable due to the high viscosity and associated syringe force or protein instability.4 These high syringe forces and stability issues make SC delivery challenging, often virtually impossible, constraining the options for delivering mAb therapeutics to either IV infusion, or administering lower doses more frequently.
Injectable biopharmaceuticals typically rely on aqueous formulations to deliver drugs and biologics, but many drugs have low solubility in water and are not stable. There are a number of conventional approaches to address these barriers but, to date, these have been insufficient. Solving the formulation and delivery challenges associated with mAbs is key to enabling mAb therapeutics to reach their full medical and market potential.
INTRODUCING XERIJECT™

Figure 1: Spray-Dryer.
XeriJect™ is a formulation technology suited for drugs and biologics consisting of large molecules such as proteins, mAbs and vaccines. XeriJect™ formulations are innovative, ready-to-use, visco-elastic pharmaceutical suspensions for various therapeutic categories that have the potential to remove many associated treatment burdens and ultimately improve patients’ lives.
Xeris has pioneered the development of injectable, visco-elastic formulations with non-Newtonian properties. These visco-elastic suspension formulations achieve maximally high drug loadings and are ready to use due to lack of particle settling on storage. Furthermore, no novel processes or excipients are required to create XeriJect™ formulations. All formulation excipients are present in currently approved formulations for SC injection.
“Using the XeriJect™ platform, ready-to-use visco-elastic suspensions with protein drug concentrations in excess of 400 mg/mL can be routinely formulated, far exceeding current aqueous formulation systems.”
To create XeriJect™ formulations, specialised drying and particle engineering techniques are employed first to create particles/powders with highly defined characteristics. The XeriJect™ platform uses proprietary spray-drying formulation processes to engineer particles of high density and a particle-size distribution amenable to highly concentrated suspensions (see Figure 1).
Spray-drying is an oft used and well understood pharmaceutical process and, in particular, aseptic spray-drying of protein therapeutics has been scaled to metric tonne quantities. Figure 2 shows an example of spray-dried particles amenable to XeriJect™ formulations.
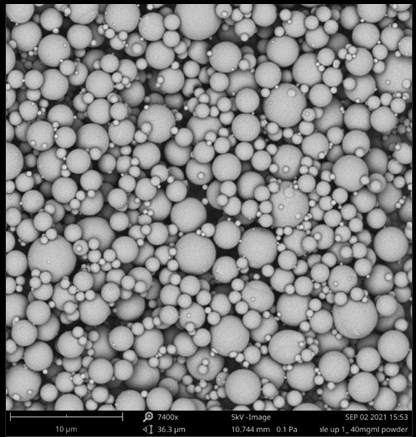
Figure 2: Electron micrograph of spray-dried particles used in XeriJect formulations.
In creating XeriJect™ formulations, spray-dried powders are then “wetted” with biocompatible diluents, creating ultra-concentrated suspension formulations (see Figure 3). At very high particle loadings, these formulations take on visco-elastic properties, allowing for maximal drug loading, but are amenable to injection via syringes and cartridges. These suspensions are highly viscous at rest, but the viscosity drops by orders of magnitude upon the application of shear.

Figure 3: Example XeriJect™ spray-dried powder, visco-elastic suspension and prefilled syringe.
Xeris uses commercially available filling technologies to fill syringes or cartridges. XeriJect™ formulations have been filled into several commercially available prefilled syringes and cartridges. Figure 3 also shows an example of a 1 mL “long” prefilled syringe filled with XeriJect™ formulation; an elegant pharmaceutical presentation.
Using the XeriJect™ platform, ready-to-use visco-elastic suspensions with protein drug concentrations in excess of 400 mg/mL can be routinely formulated, far exceeding current aqueous formulation systems with maximum achievable protein concentrations of 50–250 mg/mL.4 In certain cases, the XeriJect™ technology can produce formulations in excess of 500 mg/mL of protein therapeutic. Additionally, these highly concentrated suspensions can be delivered through relatively small needles at injection forces amenable to thumb pressure. The visco-elastic properties also allow for delivery from cartridges using standard cartridge needles.
The injectability of the XeriJect™ formulations is measured using a texture analyser. During this analysis, the break-loose force and the mean glide force of prefilled syringes or syringe cartridges are evaluated at different volumetric flow rates. Figure 4 shows an injection force profile for a XeriJect™ formulation administered using a prefilled syringe with a 27-gauge needle. The injection force of the XeriJect™ formulations is dictated by the powder-to-oil ratios, geometry of the syringe, needle gauge, and injection speed. Various syringe and needle combinations are possible with XeriJect™ depending on the application and requirements. Modifications to these parameters allow Xeris to ensure injection forces do not exceed certain limits, providing safe administration to the patient.
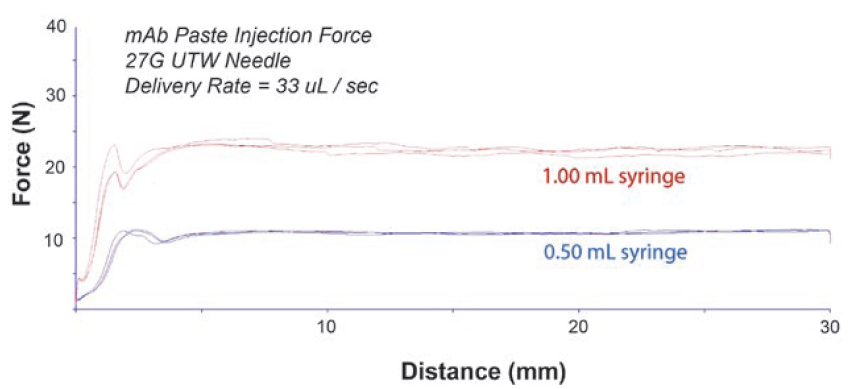
Figure 4: Injection force profile for a XeriJect™ prefilled syringe with a 27-gauge needle.
INTELLECTUAL PROPERTY
The XeriJect™ technology platform and formulations are proprietary, and supported and protected by an extensive patent estate, trade secrets, and development and manufacturing know-how, providing an opportunity for drug developers to achieve and/or maintain market exclusivity for their products.
Xeris seeks to collaborate in the development of XeriJect™ formulations and make the technology available for licensing, allowing for lengthy patent terms.
ASEPTIC MANUFACTURING
Most biologics cannot be terminally sterilised, and the highly concentrated XeriJect™ suspensions cannot not be sterile filtered. Thus, development of XeriJect™ formulations will require processing in an aseptic environment. Incoming bulk aqueous solutions of mAbs can be sterile filtered into an aseptic isolator. All of the processes used to manufacture XeriJect™ formulations are amenable to processing in an aseptic environment.
PRECLINICAL STUDIES
XeriJect™ formulations can be administered SC (or intramuscularly) using commercially available prefilled syringes, pens and pumps. Once the injected visco-elastic suspension mixes with the physiologic, aqueous environment of the subcutaneous tissue, complete dissolution of the formulation occurs rapidly, mitigating immunological risks posed by particles present in the subcutaneous space.
Figure 5 shows the pharmacokinetic profile of a XeriJect™ formulation injection compared with both an IV infusion and a SC injection of trastuzumab, in a large animal model (Yucatan minipig). Table 1 contains a list of highlighted pharmacokinetic parameters comparing injection of XeriJect™ suspensions with IV and SC administration of the approved aqueous formulation of trastuzumab drug (Herceptin®). Compared with IV infusion, XeriJect™ formulation administered SC has a longer absorption phase, though the observed elimination half-life is identical. When compared with an SC injection of aqueous trastuzumab, XeriJect™ displays similar absorption and elimination profiles with only minor differences, despite the ~20-fold higher mAb concentration in the XeriJect™ formulation. The XeriJect™ mAb formulation also shows high bioavailability, similar to aqueous IV and SC administration, indicating that, upon injection into the SC space, the XeriJect™ formulation dissolves rapidly and becomes bioavailable.
| Treatment | Dose | N | Cmax (μg/mL) |
Tmax (hr) |
AUC0-inf (μg.hr/ml) |
T½ (hr) |
| Herceptin IV | 10 mg/kg | 4 | 180 ±5 | 0.25 ±0 | 19,160 ±1,170 | 115 ±12 |
| Herceptin SC | 120 mg | 4 | 92 ±2 | 90 ±15 | 27,674 ±2,759 | 122 ±19 |
| XeriJect Trastuzumab SC | 120 mg | 4 | 92 ±2 | 48 ±0 | 32,118 ±2,587 | 140 ±5 |
Table 1: Pharmacokinetic Parameters of Trastuzumab in Yucatan Minipigs.
Neither a depot nor a sustained-release effect is observed. Thus, compared with an equal dose of aqueous mAb, XeriJect™ trastuzumab demonstrated similar pharmacokinetic profiles, allowing for confidence in undertaking XeriJect™ drug development programs.
CONCLUSION
Highly concentrated, ready-to-use formulations are required to optimise patient acceptance of mAb therapies. mAb therapeutics are increasingly dominating the biopharmaceutical landscape by market share, although patient acceptance remains a problem. Xeris’ next-generation formulation and delivery platform, XeriJect™, can enhance acceptance and accessibility by enabling the SC delivery of protein therapeutics at high doses and high stability.
The XeriJect™ technology leads to products that are easier to use for patients and caregivers, while reducing costs for payers and the healthcare system.
REFERENCES
- Ecker DM et al, “The Therapeutic Monoclonal Antibody Product Market”. BioProcess International, 2020, Vol 18(10).
- Janssen Biotech, “Darzalex (daratumumab) [FDA label]”. 2019.
- Viola M et al, “Subcutaneous delivery of monoclonal antibodies: How do we get there?”. J Control Rel, 2018, Vol 286, pp 301-314.
- Bussemer T et al, “Approaches in subcutaneous delivery of monoclonal antibodies”. Eur Pharm Rev, 2016, Vol 4, pp 26-31.

