Citation: Thompson I, “YpsoDose – Simplifying Large-Volume Patch Injections”. ONdrugDelivery, Issue 124 (Sep 2021), pp 6–8.
Ian Thompson provides the latest insights into the YpsoDose prefilled and pre-assembled patch injector as it moves into the industrialisation phase for clinical trials.
PREPARING A NEW PLATFORM FOR CLINICAL TRIALS
The YpsoDose patch injector has been developed in response to the significant increase in demand for new large-volume subcutaneous therapies. These new therapies are usually antibody-based and have payloads of up to 1000 mg that require a 5–10 mL fill volume. Therapies in this category include treatments for autoimmune diseases and orphan and rare diseases, as well as for the delivery of immuno-oncology drugs.
Various comparative studies have confirmed that patients and healthcare providers (HCPs) prefer a prefilled and pre-assembled device that increases convenience. Beyond the immediate advantages of convenience, safety, correct dosing and time saving, pharma customers particularly like that the user is unable to manipulate the drug container before or after the injection event. Ypsomed has drawn on its experience with prefilled pen injectors and autoinjectors to develop a modular and customisable platform to speed up time-to-clinic, lower up-front investment costs and lower project risks for pharma partners.
INNOVATIVE SOLUTIONS FOR SAFE AND SIMPLE HANDLING
For very infrequent injections, the number of use steps, and thus complexity, must be minimised to ensure that all users will remember the correct handling for their device even with a lengthy timespan between injections. Accordingly, simplicity and safety are key requirements for a patch injector, and this is reflected in the YpsoDose design. Being prefilled and pre-assembled, handling is reduced to two simple steps – patch and inject.
The innovative solutions that are core to the YpsoDose platform are all very closely linked:
- 10 mL Cartridge: The drug is filled into a 10 mL glass cartridge, cartriQ® from SCHOTT, which uses standard-dimensioned components, including a standard 13 mm coated stopper and a coated plunger. The stopper is protected by a sterile barrier during storage, which is removed immediately before use when the patch release liner is removed.
- Needle Unit: The needle unit contains the fluid path, including the cartridge needle and injection needle that allow the drug to be administered. Customisation of the injection needle is an important option to accommodate more viscous drugs or faster injection times. The needle unit is fully mechanical, and its functionality is controlled by the electromechanical drive mechanism.
- Sensing Patch: The capacitive sensing patch ensures that YpsoDose is correctly attached to the skin during the injection process and signals if YpsoDose is removed prematurely. Most importantly, immediately prior to injection, during the removal of the patch release liner, the needle unit’s sterile barriers are also removed.
- Electromechanical Drive: Above all, the drive mechanism and electronics provide safety, reassurance and flexibility. Safety due to direct links to the needle unit and sensing patch; reassurance to the user based on clear feedback; and flexibility to accommodate different drug fill volumes, viscosities and injection times.
YpsoDose’s functionality has been fully tested according to the relevant parts of the current draft of “ISO/FDIS 11608 Needle-based Injection Systems for Medical Use – Requirements and Test Methods”, including “Part 6: On-body Delivery Systems”.
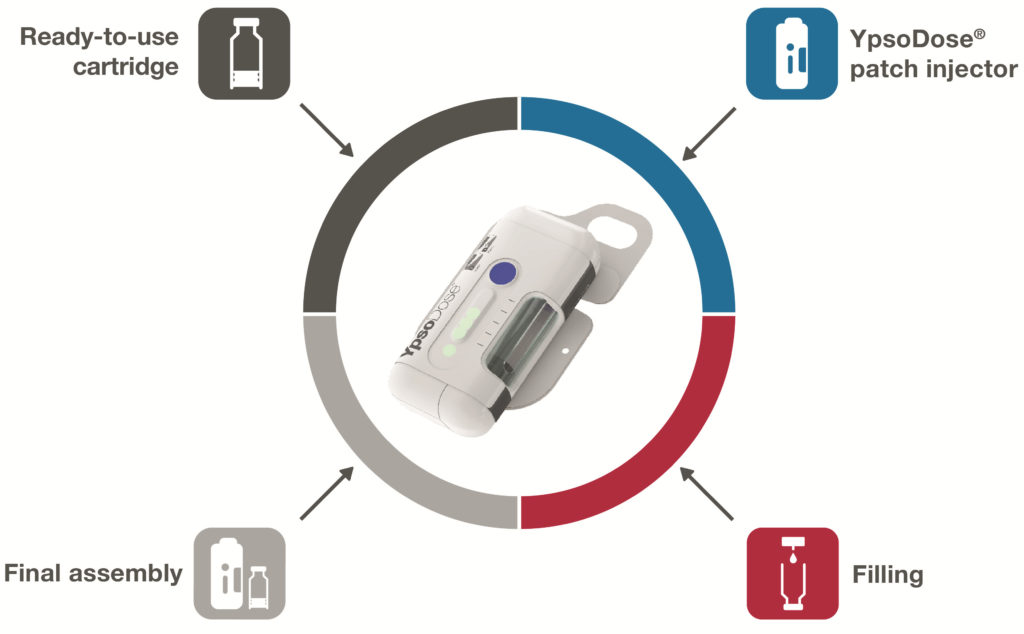
Figure 1: YpsoDose represents a collaboration between Ypsomed, for the patch injector design, SCHOTT, for the ready-to-use glass cartridge, and Lonza, for filling, characterisation and final assembly services.
COLLABORATIVE APPROACH WITH EXPERIENCED PARTNERS
In order to develop a ready-to-use 10 mL glass cartridge, it was important to build up the requisite know-how with experienced partners. In 2020 Ypsomed, SCHOTT and Lonza announced the YpsoDose patch injector collaboration (Figure 1). The three companies are co-operating closely to develop all the necessary components and manufacturing processes. The comprehensive solution includes the ready-to-use cartriQ® glass cartridge from SCHOTT as the primary packaging for the drug, the YpsoDose patch injector platform from Ypsomed and the processes for filling, assembly and testing of the final product by Lonza Drug Product Services (Figure 1).
This collaboration brings together three experts in their respective fields. All the processes and components of the solution are predeveloped, co-ordinated and tested. The 10 mL ready-to-use cartriQ® from SCHOTT requires fewer process steps during filling than conventional cartridges. Lonza Drug Product Services applies its expertise in process development, testing and characterisation, and provides filling and assembly services for YpsoDose in Stein (Switzerland) to provide short implementation times for customers. Pharma customers thus receive a tested and documented overall solution and can concentrate on their core business.
BOX 1: YPSODOSE PATCH INJECTOR OVERVIEW

Figure 2: YpsoDose, the prefilled electromechanical motor-driven patch injector.
Designing and developing a wearable patch injector is a demanding task, requiring a broad range of technology and medical device competencies. Ideally, the infrequently used patch injector should be as easy, if not easier, to use as a disposable two-step autoinjector, which is why the prefilled YpsoDose format (Figure 2) incorporates the following key technical features and benefits:
- Prefilled and pre-assembled to avoid any need for the patient or HCP to assemble or fill the drug reservoir and device
- Adheres to the skin during injection and is easy to remove after injection
- Capacitive sensing patch only allows initiation of the injection after the skin sensor has confirmed skin contact
- Automatic needle insertion at the start and retraction at the end of the injection process, and the needle is also retracted if the device is removed from the skin before the end of injection
- Electromechanical drive accommodates a range of fill volumes and viscosities, and provides a programmable and reproducible injection time and volume for each drug
- Audible and visual feedback to clearly communicate with the user before, during and after the injection
- Integrated electronics allow wireless connectivity to provide additional patient or HCP therapy support.
TRIED AND TESTED HUMAN FACTORS
Current patch injectors are generally HCP or patient-filled or -assembled; no prefilled, ready-to-use wearable devices have been approved for use by patients to date. To ensure that patch injector therapies are widely adopted for biological therapies, usability is the most important aspect that needs to be successfully tested with patients and HCPs. During the development process, YpsoDose has gone through a number of iterations and various rounds of usability and human factors (HF) testing. The final formative HF evaluation was successfully performed in early 2021, the detailed results of which will be published shortly. The main focus of the study was to test the remaining minor product changes linked to the patch/release liner functionality and the electronic user interface.
YPSODOSE – THE LATEST SELFINJECTION PRODUCT PLATFORM
For pharma companies to consider and invest in patch injectors, they need to be able to access reliable device technology and implement standard filling and final assembly processes that provide a solution that fulfils the needs of patients and HCPs. Fulfilling these requirements with well thought-out device technology will allow the patch injector market to grow significantly over the coming years and to become established as the third self-injection device class, complementing the maturing markets for pen injectors and autoinjectors. The 10 mL YpsoDose has undergone thorough internal testing and comparative studies with pharma customers and Ypsomed is committed to the successful industrialisation and commercialisation of YpsoDose as a new state-of-the-art patch injector.
Previous article
THE PATH TO COMMERCIALISATION FOR WEARABLE DRUG DELIVERY DEVICESNext article
PHOTOSTABILITY TESTS OF ANTIBODY DRUGS
